Li-ion diffusional insights and temperature dependent electrical & dielectric properties of LiNi1/3Mn1/3Co1/3PO4 electrodes
IF 4.3
3区 材料科学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
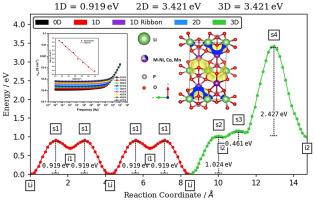
Enhancing the electrical conductivity of the cathode is crucial for improving the overall performance of modern lithium-ion (Li-ion) batteries. In this study, a facile sol-gel thermolysis technique was employed to synthesize LiNi1/3Mn1/3Co1/3PO4, using ethylene-di-amine-tetra-acetic acid (EDTA) as the chelating agent. This method ensures a uniform distribution of metal ions, which is vital for achieving consistent and reliable material properties. This study employed solid-state impedance to investigate the electrical conductivity and Bond Valence Site Energy (BVSE) analysis to find the Li-ion trajectory within the proposed LiNi1/3Mn1/3Co1/3PO4 compound. Especially, the conductivity analysis revealed the small polaron hopping mechanism, a process in which charge carriers move through the crystal lattice by hopping from one localized site to another. The conductance spectra revealed that at 473 K, the compound exhibited a high DC conductivity of 6.88 x 10⁻⁶ S cm⁻1, which indicates that the material maintains a reasonable electrical conductivity at elevated temperatures for high-performance battery applications. The activation energy for conduction, determined from the Arrhenius plot, was found to be 0.14 eV, suggesting that the compound has a relatively low energy barrier for charge carrier movement. The half-cell of LiNi1/3Mn1/3Co1/3PO4 as cathode demonstrates an initial discharge capacity of 94 mAh g−1 at 0.1 C in the potential window from 2 to 4.8 V vs. Li/Li+. The bond valence site energy (BVSE) modeling provided critical insights into the Li-ion migration within the cathode material. The analysis revealed a one-dimensional (1D) Li⁺ migration barrier of 0.919 eV and a hopping distance of 1.86 Å. These findings infer the potential of LiNi1/3Mn1/3Co1/3PO4 as a promising candidate for Li-ion energy storage applications.
镍1/3锰1/3钴1/3PO4锂离子电极的锂离子扩散见解以及与温度相关的电学和介电特性
增强阴极的导电性对于提高现代锂离子(Li-ion)电池的整体性能至关重要。本研究采用简便的溶胶-凝胶热解技术合成了 LiNi1/3Mn1/3Co1/3PO4,并使用乙二胺四乙酸(EDTA)作为螯合剂。这种方法可确保金属离子的均匀分布,这对实现稳定可靠的材料特性至关重要。本研究采用固态阻抗来研究电导率,并通过键价位能(BVSE)分析来寻找锂离子在拟议的 LiNi1/3Mn1/3Co1/3PO4 复合物中的运动轨迹。特别是,电导率分析揭示了小极子跳跃机制,即电荷载流子通过从一个局部位点跳跃到另一个局部位点在晶格中移动的过程。电导率光谱显示,在 473 K 时,该化合物表现出 6.88 x 10-⁶ S cm-1 的高直流电导率,这表明该材料在高温下仍能保持合理的电导率,适用于高性能电池应用。根据阿伦尼乌斯图确定的传导活化能为 0.14 eV,表明该化合物的电荷载流子运动能量势垒相对较低。以 LiNi1/3Mn1/3Co1/3PO4 为阴极的半电池在 0.1 摄氏度、2 至 4.8 V 的电位窗口内对 Li/Li+ 的初始放电容量为 94 mAh g-1。键价位能(BVSE)建模为了解锂离子在阴极材料中的迁移提供了重要依据。分析结果表明,一维(1D)锂离子迁移势垒为 0.919 eV,跳跃距离为 1.86 Å。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.80
自引率
2.50%
发文量
605
审稿时长
40 days
期刊介绍:
The Journal of Physics and Chemistry of Solids is a well-established international medium for publication of archival research in condensed matter and materials sciences. Areas of interest broadly include experimental and theoretical research on electronic, magnetic, spectroscopic and structural properties as well as the statistical mechanics and thermodynamics of materials. The focus is on gaining physical and chemical insight into the properties and potential applications of condensed matter systems.
Within the broad scope of the journal, beyond regular contributions, the editors have identified submissions in the following areas of physics and chemistry of solids to be of special current interest to the journal:
Low-dimensional systems
Exotic states of quantum electron matter including topological phases
Energy conversion and storage
Interfaces, nanoparticles and catalysts.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


