DNA-templated nanosheets for enhanced chemodynamic therapy and gene therapy to inhibit tumor recurrence and metastasis
IF 5.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
Recurrence and metastasis stand as the primary contributors to mortality among patients with triple-negative breast cancer post-surgery, presenting a formidable clinical obstacle. Chemodynamic therapy (CDT), leveraging metal-ion-mediated Fenton-like reactions within the tumor microenvironment (TME), emerges as a promising avenue for addressing cancer metastasis. Despite recent progress, challenges such as tumor cell antioxidant defenses and epithelial-mesenchymal transition (EMT) impede the efficacy of CDT. Here, we introduce a novel approach using DNA-templated nanosheets (Dz-MnO2) that combine the functions of Mn2+-mediated CDT and DNAzyme-mediated gene therapy to suppress tumor growth and metastasis. The Dz-MnO2 nanosheets respond effectively to the TME, releasing Mn2+ and DNAzyme. The DNAzyme exhibits mRNA cleavage activity, specifically targeting oncogenic transcripts to reduce tumor progression. Mn2+ not only facilitates a Fenton-like reaction, enhancing the chemodynamic treatment effect, but also serves as a cofactor for DNAzyme, improving its catalytic efficiency. Concurrently, the nanosheets robustly silence the Twist1 gene, mitigating the EMT process and reinforcing CDT efficacy by suppressing apoptosis resistance. Results indicate that Dz-MnO2 nanosheets efficiently polarize M2-tumor-associated macrophages (TAMs) into M1-TAMs by locally mitigating tumor hypoxia via catalyzing the decomposition of H2O2 into O2. This collaborative strategy presents a promising approach to enhance CDT, effectively inhibiting tumor recurrence and metastasis.
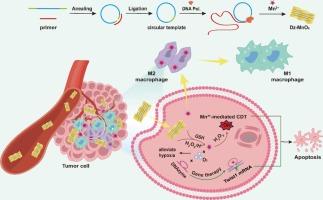
用于增强化学动力疗法和基因疗法以抑制肿瘤复发和转移的 DNA 模板纳米片。
复发和转移是导致三阴性乳腺癌患者术后死亡的主要原因,给临床治疗带来了巨大障碍。化学动力疗法(CDT)利用金属离子介导的肿瘤微环境(TME)中的芬顿样反应,成为解决癌症转移问题的一条前景广阔的途径。尽管最近取得了进展,但肿瘤细胞抗氧化防御和上皮-间质转化(EMT)等挑战阻碍了 CDT 的疗效。在这里,我们介绍了一种使用DNA模板纳米片(Dz-MnO2)的新方法,它结合了Mn2+介导的CDT和DNA酶介导的基因治疗功能,可抑制肿瘤生长和转移。Dz-MnO2纳米片能有效响应TME,释放出Mn2+和DNA酶。DNA 酶具有切割 mRNA 的活性,可特异性地靶向致癌转录本,从而减少肿瘤的进展。Mn2+ 不仅能促进芬顿反应,增强化学动力学治疗效果,还能作为 DNA 酶的辅助因子,提高其催化效率。同时,纳米片还能抑制 Twist1 基因,减轻 EMT 过程,并通过抑制细胞凋亡抵抗来增强 CDT 的疗效。研究结果表明,Dz-MnO2 纳米片通过催化 H2O2 分解为 O2,局部缓解肿瘤缺氧,从而有效地将 M2 肿瘤相关巨噬细胞(TAMs)极化为 M1-TAMs。这一合作策略为加强 CDT、有效抑制肿瘤复发和转移提供了一种前景广阔的方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
10.70
自引率
8.60%
发文量
951
审稿时长
72 days
期刊介绍:
The International Journal of Pharmaceutics is the third most cited journal in the "Pharmacy & Pharmacology" category out of 366 journals, being the true home for pharmaceutical scientists concerned with the physical, chemical and biological properties of devices and delivery systems for drugs, vaccines and biologicals, including their design, manufacture and evaluation. This includes evaluation of the properties of drugs, excipients such as surfactants and polymers and novel materials. The journal has special sections on pharmaceutical nanotechnology and personalized medicines, and publishes research papers, reviews, commentaries and letters to the editor as well as special issues.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


