DES/O microemulsion for solubilizing and delivering curcumin via the nasal administration to treat acute asthma
IF 5.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
As a natural small molecule drug derived from turmeric, curcumin is known for its good biosafety and a range of beneficial effects, including anti-inflammatory, anti-spasmodic, antioxidant, antibacterial, anti-tumor, and neuroprotective effects. Even though, its insolubility in water and instability severely limit its bioavailability and clinical applications. The present study developed a deep eutectic solvent (DES) in oil microemulsion (DES/O-ME) system loaded with Cur for intranasal administration, aiming to enhance both the solubility and permeability of Cur to significantly increase its bioavailability. The project first constructed and screened the optimal choline chloride-based DES. Subsequently, the DES/O-ME system was prepared and optimized to achieve uniform particle size and stability using a pseudo-ternary phase diagram and electrical conductivity measurements. The resulting DES/O-ME system was thoroughly evaluated for its solubilization efficacy, permeability, mucosal cytotoxicity, and stability. Additionally, the therapeutic efficacy of the Cur-DES/O-ME was assessed in vivo using a murine model of allergic asthma. This comprehensive evaluation highlights the potential of the DES/O-ME system as a promising intranasal drug delivery platform to overcome the limitations of curcumin’s bioavailability and clinical application.
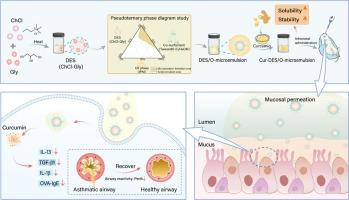
DES/O 微乳液,用于通过鼻腔给药增溶和输送姜黄素,治疗急性哮喘。
姜黄素是从姜黄中提取的一种天然小分子药物,以其良好的生物安全性和一系列有益作用而闻名,包括抗炎、抗痉挛、抗氧化、抗菌、抗肿瘤和神经保护作用。尽管如此,其不溶于水和不稳定性严重限制了其生物利用度和临床应用。本研究开发了一种深共晶溶剂(DES)油微乳剂(DES/O-ME)系统,装载莪术用于鼻内给药,旨在增强莪术的溶解性和渗透性,从而显著提高其生物利用度。该项目首先构建并筛选了最佳氯化胆碱基 DES。随后,制备并优化了 DES/O-ME 系统,利用伪三元相图和电导率测量法实现了均匀的粒度和稳定性。对所制备的 DES/O-ME 系统的增溶效果、渗透性、粘膜细胞毒性和稳定性进行了全面评估。此外,还利用过敏性哮喘小鼠模型对 Cur-DES/O-ME 的治疗效果进行了体内评估。这项综合评估凸显了 DES/O-ME 系统作为鼻内给药平台的潜力,有望克服姜黄素生物利用度和临床应用的局限性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
10.70
自引率
8.60%
发文量
951
审稿时长
72 days
期刊介绍:
The International Journal of Pharmaceutics is the third most cited journal in the "Pharmacy & Pharmacology" category out of 366 journals, being the true home for pharmaceutical scientists concerned with the physical, chemical and biological properties of devices and delivery systems for drugs, vaccines and biologicals, including their design, manufacture and evaluation. This includes evaluation of the properties of drugs, excipients such as surfactants and polymers and novel materials. The journal has special sections on pharmaceutical nanotechnology and personalized medicines, and publishes research papers, reviews, commentaries and letters to the editor as well as special issues.
文献相关原料
| 公司名称 | 产品信息 | 采购帮参考价格 |
|---|---|---|
| 上海源叶 |
Betaine
|

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


