Synthetic studies on the tetrasubstituted D-ring of cystobactamids lead to potent terephthalic acid antibiotics
IF 5.9
2区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
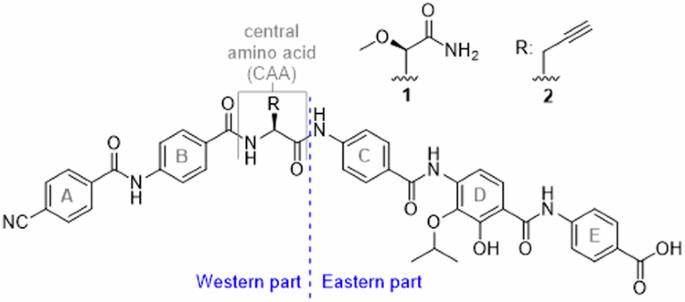
Novel scaffolds for broad-spectrum antibiotics are rare and in strong demand because of the increase in antimicrobial resistance. The cystobactamids, discovered from myxobacterial sources, have a unique hexapeptidic scaffold with five arylamides and possess potent, resistance-breaking properties. This study investigates the role of the central D-ring pharmacophore in cystobactamids, a para-aminobenzoic acid (PABA) moiety that is additionally substituted by hydroxy and isopropoxy functions. We varied the two oxygenated substituents and replaced both amide connectors with bioisosteres. Synthetic routes were developed that included metal-mediated aromatic functionalization or heterocycle formations, leading to 19 novel analogues. The antibiotic efficacy of all analogues was determined against bacteria from the ESKAPE pathogen panel. While the replacement and the repositioning of hydroxy and isopropoxy substituents was not advantageous, exchanging PABA by terephthalic acid amides led to the highly potent analogue 42 with broad-spectrum activity, insensitivity towards AlbD-mediated degradation and promising pharmacokinetic properties in mice. The study highlights the steep structure-activity relationships in the tetrasubstituted D-ring and a surprisingly favorable reversion of the amide connecting C and D. Novel scaffolds for broad-spectrum antibiotics are rare and in strong demand because of the increase in antimicrobial resistance. Here, the authors assess the role of the central D ring pharmacophore in cystobactamids, and develop a potent broad-spectrum antibiotic by exchanging a para-aminobenzoic acid with a terephthalic acid amide.
通过对四取代 D 环的胱杆菌酰胺的合成研究,产生了强效对苯二甲酸抗生素。
由于抗菌药耐药性的增加,广谱抗生素的新型支架十分罕见,而且需求量很大。从霉菌中发现的胱内酰胺类药物具有独特的六肽支架,包含五个芳基酰胺,具有强效的抗药性。本研究探讨了胱内酰胺类药物的中心 D 环药理作用,即对氨基苯甲酸(PABA)分子,该分子还被羟基和异丙氧基功能取代。我们改变了这两个含氧取代基,并用生物异构体取代了两个酰胺连接体。我们开发了包括金属介导的芳香族官能化或杂环形成在内的合成路线,最终得到了 19 种新型类似物。所有类似物对 ESKAPE 病原体样本中细菌的抗生素疗效均已确定。虽然羟基和异丙氧基取代基的置换和重新定位没有优势,但用对苯二甲酸酰胺置换 PABA 可得到具有广谱活性、对 AlbD 介导的降解不敏感以及在小鼠体内具有良好药代动力学特性的强效类似物 42。该研究强调了四取代 D 环陡峭的结构-活性关系,以及连接 C 和 D 的酰胺令人惊讶的有利还原。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Communications Chemistry
Chemistry-General Chemistry
CiteScore
7.70
自引率
1.70%
发文量
146
审稿时长
13 weeks
期刊介绍:
Communications Chemistry is an open access journal from Nature Research publishing high-quality research, reviews and commentary in all areas of the chemical sciences. Research papers published by the journal represent significant advances bringing new chemical insight to a specialized area of research. We also aim to provide a community forum for issues of importance to all chemists, regardless of sub-discipline.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


