Phase II study of long-course chemoradiotherapy followed by consolidation chemotherapy as total neoadjuvant therapy in locally advanced rectal cancer in Japan: ENSEMBLE-2
Abstract
Aim
To evaluate the feasibility and safety of total neoadjuvant therapy with long-course chemoradiotherapy followed by consolidation chemotherapy in Japanese patients with locally advanced rectal cancer.
Methods
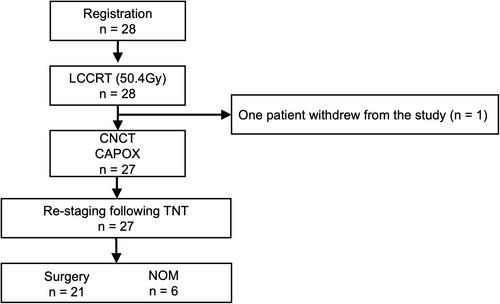
This prospective, multicenter, single-arm, phase II trial was conducted at 10 centers. The eligibility criteria included age ≥20 y, locally advanced rectal cancer within 12 cm of the anal verge, and cT3-4N0M or TanyN+M0 at diagnosis, enabling curative resection. The protocol treatment was capecitabine (1650 mg/m2/day)-based long-course chemoradiotherapy (50.4 Gy/28 fractions) and consolidation chemotherapy (CAPOX, four courses) followed by total mesorectal excision. Nonoperative management was allowed if a clinical complete response was achieved. The primary endpoint was the pathologic complete response rate.
Results
Among 28 enrolled patients (19 men, 9 women; median age, 69.5 [41–79] y), the long-course chemoradiotherapy and consolidation chemotherapy completion rates were 100% and 96.4%, respectively. The clinical responses included clinical complete response, (35.7%, 10/28), near-complete response (28.6%, 8/28), and incomplete response (32.1%, 9/28). Total mesorectal excision and nonoperative management were performed in 21 and six patients, respectively. The final analysis included 21 patients. Five patients (23.8% [90% confidence interval 11.8%–41.8%]) achieved pathologic complete response, while 10 of 28 patients (35.7%) achieved a pathological complete response or a sustained clinical complete response. No treatment-related deaths occurred. Grade ≥3 adverse events included diarrhea (7.1%) and leukopenia (7.1%).
Conclusion
ENSEMBLE-2 demonstrated comparable pathologic complete response rates and well-tolerated safety of total neoadjuvant therapy with long-course chemoradiotherapy followed by consolidation chemotherapy in Japanese patients with locally advanced rectal cancer.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


