Nanoparticle-mediated Klotho gene therapy prevents acute kidney injury to chronic kidney disease transition through regulating PPARα signaling in renal tubular epithelial cells
IF 12.8
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
Klotho is an anti-aging protein produced primarily by tubular epithelial cells (TECs). Down-regulated expression of Klotho in injured TECs plays a key pathogenic role in promoting acute kidney injury (AKI) to chronic kidney disease (CKD) transition, yet therapeutic approaches targeting the restoration of renal Klotho levels remain challenging for clinical application. Here, we synthesize polydopamine-polyethylenimine-l-serine-Klotho plasmid nanoparticles (PPSK NPs), which can safely and selectively deliver the Klotho gene to the injured TECs through binding kidney injury molecule-1 and maintain the expression of Klotho protein. In vitro, PPSK NPs effectively reduce the hypoxia-reoxygenation-induced reactive oxygen species production and fibrotic gene expression. In the unilateral ischemia-reperfusion injury- and folic acid-induced AKI-CKD transition mouse models, a single low-dose injection of PPSK NPs is sufficient to preserve the normal kidney architecture and prevent renal fibrosis. Mechanismly, the protective effect of PPSK NPs relies on upregulating a key molecule peroxisome proliferator-activated receptor alpha (PPARα) via the inhibition of p38 and JNK phosphorylation, which in turn improves tubular fatty acid beta-oxidation and reduces renal lipid accumulation, thereby protecting against kidney fibrosis. In conclusion, our results highlight the translational potential of nanoparticle-based Klotho gene therapy in preventing the AKI-CKD transition.
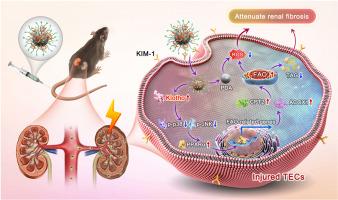
纳米粒子介导的 Klotho 基因疗法通过调节肾小管上皮细胞中的 PPARα 信号,防止急性肾损伤向慢性肾病转变。
Klotho是一种抗衰老蛋白,主要由肾小管上皮细胞(TEC)产生。在急性肾损伤(AKI)向慢性肾病(CKD)转变的过程中,受伤的肾小管上皮细胞中 Klotho 的表达下调起着关键的致病作用,然而针对恢复肾小管上皮细胞 Klotho 水平的治疗方法在临床应用中仍面临挑战。在这里,我们合成了聚多巴胺-聚乙烯亚胺-丝氨酸-Klotho质粒纳米颗粒(PPSK NPs),它能通过结合肾损伤分子-1将Klotho基因安全、选择性地传递到损伤的TECs,并维持Klotho蛋白的表达。在体外,PPSK NPs 能有效减少缺氧-复氧诱导的活性氧生成和纤维化基因表达。在单侧缺血再灌注损伤和叶酸诱导的 AKI-CKD 转换小鼠模型中,单次低剂量注射 PPSK NPs 就足以保护正常的肾脏结构并防止肾脏纤维化。从机制上看,PPSK NPs 的保护作用依赖于通过抑制 p38 和 JNK 磷酸化上调关键分子过氧化物酶体增殖激活受体α(PPARα),进而改善肾小管脂肪酸 beta 氧化,减少肾脏脂质积累,从而防止肾脏纤维化。总之,我们的研究结果凸显了基于纳米粒子的 Klotho 基因疗法在预防 AKI-CKD 转化方面的转化潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Biomaterials
工程技术-材料科学:生物材料
CiteScore
26.00
自引率
2.90%
发文量
565
审稿时长
46 days
期刊介绍:
Biomaterials is an international journal covering the science and clinical application of biomaterials. A biomaterial is now defined as a substance that has been engineered to take a form which, alone or as part of a complex system, is used to direct, by control of interactions with components of living systems, the course of any therapeutic or diagnostic procedure. It is the aim of the journal to provide a peer-reviewed forum for the publication of original papers and authoritative review and opinion papers dealing with the most important issues facing the use of biomaterials in clinical practice. The scope of the journal covers the wide range of physical, biological and chemical sciences that underpin the design of biomaterials and the clinical disciplines in which they are used. These sciences include polymer synthesis and characterization, drug and gene vector design, the biology of the host response, immunology and toxicology and self assembly at the nanoscale. Clinical applications include the therapies of medical technology and regenerative medicine in all clinical disciplines, and diagnostic systems that reply on innovative contrast and sensing agents. The journal is relevant to areas such as cancer diagnosis and therapy, implantable devices, drug delivery systems, gene vectors, bionanotechnology and tissue engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


