Biomimetic Chlorosomes: Oxygen-Independent Photocatalytic Nanoreactors for Efficient Combination Photoimmunotherapy
IF 27.4
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Photocatalytic therapy for hypoxic tumors often suffers from inefficiencies due to its dependence on oxygen and the risk of uncontrolled activation. Inspired by the oxygen-independent and precisely regulated photocatalytic functions of natural light-harvesting chlorosomes, chlorosome-mimetic nanoreactors, termed Ru-Chlos, are engineered by confining the aggregation of photosensitive ruthenium-polypyridyl-silane monomers. These Ru-Chlos exhibit markedly enhanced photocatalytic performance compared to their monomeric counterparts under acidic conditions, while notably bypassing the consumption of oxygen or hydrogen peroxide. The photocatalytic activity of Ru-Chlos is finely tunable through light-responsive disassembly of the Ru-bridged matrix, with tunability governed by pre-irradiation duration. Utilization of Ru-Chlos loading prodrug [2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)] (ABTS) for phototherapy facilitates the generation of toxic radicals (oxABTS) and the photocatalytic conversion of endogenous NADH to NAD+, inducing oxidative stress in hypoxic cancer cells. Simultaneously, the light-responsive degradation of Ru-Chlos produces Ru-based toxins that further contribute to the therapeutic effect. This dual-action mechanism elicits potent immunogenic cell death effects and significantly enhances antitumor efficacy with the aid of a PD-l blockade. These biomimetic chlorosomes highlight their potential to advance oxygen-independent photocatalytic nanoreactors with controlled activity for novel cancer photoimmunotherapy strategies.
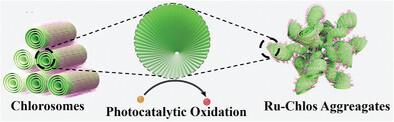
仿生氯小体:用于高效复合光免疫疗法的氧依赖性光催化纳米反应器
针对缺氧性肿瘤的光催化疗法往往因其对氧气的依赖性和活化失控的风险而效率低下。受不依赖氧气且可精确调节的天然采光叶绿体光催化功能的启发,通过限制光敏钌-聚吡啶基硅烷单体的聚集,设计出了叶绿体模拟纳米反应器,称为 Ru-Chlos。与酸性条件下的单体相比,这些 Ru-Chlos 的光催化性能明显增强,同时明显绕过了氧气或过氧化氢的消耗。Ru-Chlos 的光催化活性可通过 Ru 桥接基质的光响应分解进行微调,其可调性受预辐照持续时间的制约。利用 Ru-Chlos 加载原药[2,2′-氮杂双(3-乙基苯并噻唑啉-6-磺酸)](ABTS)进行光疗,可促进有毒自由基(oxABTS)的生成和内源性 NADH 向 NAD+ 的光催化转化,从而诱导缺氧癌细胞产生氧化应激。与此同时,Ru-Chlos 的光反应降解产生了 Ru 基毒素,进一步促进了治疗效果。这种双重作用机制可产生强大的免疫细胞死亡效应,并在 PD-l 抑制剂的帮助下显著提高抗肿瘤疗效。这些仿生氯小体凸显了它们在推进具有可控活性的不依赖氧气的光催化纳米反应器方面的潜力,可用于新型癌症光免疫疗法策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Advanced Materials
工程技术-材料科学:综合
CiteScore
43.00
自引率
4.10%
发文量
2182
审稿时长
2 months
期刊介绍:
Advanced Materials, one of the world's most prestigious journals and the foundation of the Advanced portfolio, is the home of choice for best-in-class materials science for more than 30 years. Following this fast-growing and interdisciplinary field, we are considering and publishing the most important discoveries on any and all materials from materials scientists, chemists, physicists, engineers as well as health and life scientists and bringing you the latest results and trends in modern materials-related research every week.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


