A Multi-Enzyme Nanocascade to Target Disease-Relevant Metabolites
IF 13
2区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Metabolic processes in living organisms depend on the synergistic actions of enzymes working in proximity and in concert, catalyzing reactions effectively while regulating the formation of metabolites. This enzyme synergy offers promising therapeutic application for diseases such as alcohol intoxication, cancer, and hyperinflammation. Despite their potential, the clinical translation of enzyme cascades is restricted by challenges including poor enzyme stability, short half-life, and a lack of delivery strategies that maintain enzyme proximity. In this study, multi-enzyme nanocascades synthesized are developed through in situ atom transfer radical polymerization using a zwitterionic monomer. This method markedly enhances enzyme stability and proximity, thereby prolonging their circulation half-life after systemic administration. It is demonstrated that the nanocascades of uricase and catalase effectively reduce uric acid levels without excessive hydrogen peroxide production, providing a potential antidote for hyperuricemia. Moreover, in a murine breast cancer model, the nanocascades of glucose oxidase and catalase inhibited tumor progression and enhanced the therapeutic efficacy of doxorubicin. The prolonged circulation and promoted reaction efficacy of these nanocascades underscore their substantial potential in enzyme replacement therapy and the treatment of various diseases.
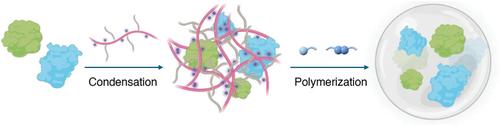
针对疾病相关代谢物的多酶纳米级联
生物体内的新陈代谢过程依赖于酶的协同作用,这些酶相互靠近并协同工作,在有效催化反应的同时调节代谢物的形成。这种酶的协同作用为治疗酒精中毒、癌症和炎症等疾病带来了希望。尽管酶级联具有潜力,但由于酶稳定性差、半衰期短以及缺乏保持酶接近性的递送策略等挑战,酶级联的临床转化受到限制。在本研究中,通过使用齐聚物单体进行原位原子转移自由基聚合,合成了多酶纳米级联。这种方法显著提高了酶的稳定性和接近性,从而延长了酶在全身给药后的循环半衰期。实验证明,尿酸酶和过氧化氢酶的纳米级联能有效降低尿酸水平,而不会产生过多的过氧化氢,为高尿酸血症提供了一种潜在的解毒剂。此外,在小鼠乳腺癌模型中,葡萄糖氧化酶和过氧化氢酶纳米级联抑制了肿瘤的进展,并增强了多柔比星的疗效。这些纳米级联可延长循环时间并促进反应效力,这凸显了它们在酶替代疗法和治疗各种疾病方面的巨大潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Small
工程技术-材料科学:综合
CiteScore
17.70
自引率
3.80%
发文量
1830
审稿时长
2.1 months
期刊介绍:
Small serves as an exceptional platform for both experimental and theoretical studies in fundamental and applied interdisciplinary research at the nano- and microscale. The journal offers a compelling mix of peer-reviewed Research Articles, Reviews, Perspectives, and Comments.
With a remarkable 2022 Journal Impact Factor of 13.3 (Journal Citation Reports from Clarivate Analytics, 2023), Small remains among the top multidisciplinary journals, covering a wide range of topics at the interface of materials science, chemistry, physics, engineering, medicine, and biology.
Small's readership includes biochemists, biologists, biomedical scientists, chemists, engineers, information technologists, materials scientists, physicists, and theoreticians alike.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


