Strong Ion-Dipole Interactions for Stable Zinc-Ion Batteries with Wide Temperature Range
IF 18.5
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Aqueous zinc-ion batteries are widely recognized as promising alternatives to lithium batteries due to their excellent safety, environmental compatibility, and cost-effectiveness. Nonetheless, the formation of dendrites, corrosion, and undesirable side reactions on the zinc surface pose significant challenges to the cycling stability of zinc-ion batteries. In this study, polar propylene carbonate (PC) is paired with tetrafluoroborate anions to establish a strong ion-dipole interaction. Strong ion-dipole interaction can not only alter the solvation structure of zinc ions but also facilitate the formation of a dynamic double electric layer on the surface of the zinc electrode, suppressing the formation of ZnF2 interface and carbonate, thereby facilitating uniform zinc ion deposition, and consequently improving battery cycling stability over a broad temperature range. Concretely, the formulated electrolyte enhances the cycling stability of the battery over a wide temperature range of −30 to 40 °C, accompanied by a capacity retention of ≈100% even after 10 000 cycles at −30 °C. The symmetrical battery utilizing this electrolyte exhibits stable cycling performance for over 1200 h at 25 °C and 1900 h at −30 °C, respectively. The findings provide a promising direction for the development of long-cycle batteries capable of operating over a wide temperature range.
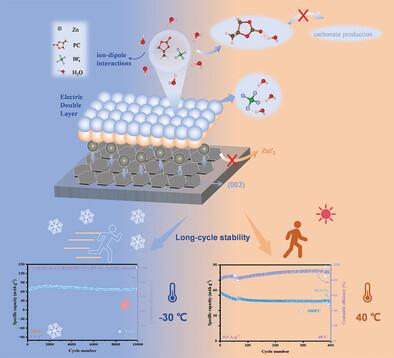
强离子-偶极子相互作用实现宽温度范围的稳定锌-离子电池
锌离子水电池因其出色的安全性、环境兼容性和成本效益,被广泛认为是锂电池的理想替代品。然而,锌表面枝晶的形成、腐蚀和不良副反应对锌离子电池的循环稳定性提出了重大挑战。在本研究中,极性碳酸丙烯酯(PC)与四氟硼酸阴离子配对,以建立强离子-偶极相互作用。强离子-偶极子相互作用不仅能改变锌离子的溶解结构,还能促进锌电极表面动态双电层的形成,抑制 ZnF2 界面和碳酸盐的形成,从而促进锌离子的均匀沉积,进而提高电池在宽温度范围内的循环稳定性。具体而言,配制的电解液提高了电池在 -30 至 40 °C 宽温度范围内的循环稳定性,即使在 -30 °C 下循环 10 000 次,容量保持率也≈100%。使用这种电解质的对称电池在 25 °C和-30 °C下分别表现出超过 1200 小时和 1900 小时的稳定循环性能。这些发现为开发能够在宽温度范围内工作的长循环电池提供了一个很有前景的方向。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Advanced Functional Materials
工程技术-材料科学:综合
CiteScore
29.50
自引率
4.20%
发文量
2086
审稿时长
2.1 months
期刊介绍:
Firmly established as a top-tier materials science journal, Advanced Functional Materials reports breakthrough research in all aspects of materials science, including nanotechnology, chemistry, physics, and biology every week.
Advanced Functional Materials is known for its rapid and fair peer review, quality content, and high impact, making it the first choice of the international materials science community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


