Nitrite-Mediated Pulsed Electrocatalytic Nitrate Reduction to Ammonia over Co@Cu NW with Dual Active Sites
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
As a potential alternative to the Haber–Bosch process for ammonia (NH3) synthesis, the electrocatalytic nitrate reduction reaction (NO3RR) has attracted extensive attention. The electrocatalytic conversion of NO3– to NH3 involves a complex 8e– reaction with various byproducts. By decomposing the overall reaction into a 2e– process from NO3– to NO2– and a 6e– process from NO2– to NH3, the two-step reaction can be strategically optimized to achieve efficient tandem catalysis. This work developed a NO2–-mediated pulsed electrocatalytic NO3RR by Co@Cu nanowire (NW) with dual active sites of the Co phase and Cu phase. The Cu phase rapidly accumulates NO2– at low potentials, while the Co phase efficiently converts NO2– to NH3 at high potentials, completing a time-separated tandem catalytic reaction. Ultimately, the Co@Cu NW achieved a maximum NH3 yield rate of 5148.6 μg·h–1·cm–2 and a maximum Faraday efficiency of 88.6% under pulsed potentials of −0.2 and −0.7 V versus the reversible hydrogen electrode in an electrolyte of 0.5 M SO42– and 0.1 M NO3–. Furthermore, in situ reflection absorption imaging and in situ total internal reflection imaging revealed that the pulsed strategy effectively enhances the utilization of NO2– and suppresses competitive hydrogen evolution reaction, thereby improving NO3RR performance.
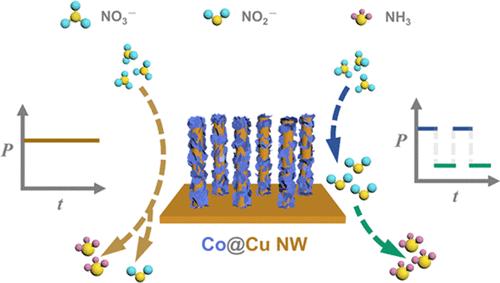
在具有双活性位点的 Co@Cu NW 上通过脉冲电催化将亚硝酸盐还原成氨气
作为哈伯-博什合成氨(NH3)工艺的潜在替代方案,电催化硝酸盐还原反应(NO3RR)引起了广泛关注。电催化将 NO3- 转化为 NH3 的过程涉及一个复杂的 8e- 反应和各种副产物。通过将整个反应分解为从 NO3- 到 NO2- 的 2e- 过程和从 NO2- 到 NH3 的 6e- 过程,可以对两步反应进行战略性优化,从而实现高效串联催化。这项研究利用具有钴相和铜相双活性位点的 Co@Cu 纳米线(NW)开发了一种以 NO2 为介导的脉冲电催化 NO3RR。Cu 相在低电位时迅速积累 NO2-,而 Co 相在高电位时则有效地将 NO2-转化为 NH3,完成一个时间分离的串联催化反应。最终,在 0.5 M SO42- 和 0.1 M NO3- 电解质中,Co@Cu NW 与可逆氢电极相比,在-0.2 V 和 -0.7 V 脉冲电位下的最大 NH3 产率为 5148.6 μg-h-1-cm-2,最大法拉第效率为 88.6%。此外,原位反射吸收成像和原位全内反射成像显示,脉冲策略有效地提高了 NO2- 的利用率,抑制了竞争性氢进化反应,从而提高了 NO3RR 的性能。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


