Oxidation Kinetics of Magnetite Nanoparticles: Blocking Effect of Surface Ligands and Implications for the Design of Magnetic Nanoheaters
IF 7.2
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
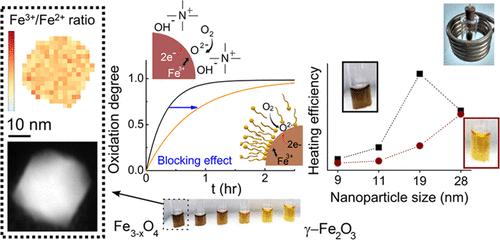
Magnetite (Fe3O4) nanoparticles (NPs) are nowadays extensively used in biomedical, environmental, and catalytic applications. However, magnetite is known to oxidize to maghemite (γ-Fe2O3), leading to changes in the physical properties of the NPs. The factors that modulate such transformation and, particularly, the role of surface capping are often overlooked. In this work, we have studied monodisperse Fe3O4 NPs synthesized by organic phase methods with sizes between 9 and 28 nm and we report on the oxidation kinetics of stable NP colloids in organic and aqueous media. The fraction of Fe3O4 in the as-prepared NPs was found to depend on their size but, in contrast to usual assumptions, monochromated electron energy loss spectroscopy results reveal that the Fe2+ concentration is homogeneous across nonstoichiometric nanocrystals, without evidence of a core/shell structure with a γ-Fe2O3 outer layer. Additionally, we show that typical ligand-exchange procedures employed to remove oleate capping from the surface lead to partially oxidized NPs, indicating that surface ligands play a key role in hindering the oxidation reaction. To elucidate the effect of the capping agent in the redox transformation, we demonstrate that the oxidation process is notably slowed for NPs with increasing oleate coverages. Then, we interpreted these findings quantitatively by considering the coupling between the surface reactivity and diffusion of cations within the oxide. Finally, we demonstrate the remarkable impact of the oxidation process on the magnetic properties of the NPs and on their heating efficiencies under radio frequency magnetic fields. Overall, our results shed light on the importance of the design of iron oxide-based nanomaterials with increased chemical stability and greater control of their physical properties, which are key aspects for their successful application.
磁铁矿纳米颗粒的氧化动力学:表面配体的阻滞效应及其对磁性纳米加热器设计的影响
磁铁矿(Fe3O4)纳米粒子(NPs)如今被广泛应用于生物医学、环境和催化领域。然而,众所周知,磁铁矿会氧化成镁铁矿(γ-Fe2O3),从而导致纳米粒子的物理性质发生变化。调节这种转变的因素,尤其是表面覆盖层的作用往往被忽视。在这项工作中,我们研究了通过有机相方法合成的单分散 Fe3O4 NPs,其尺寸介于 9 纳米和 28 纳米之间,并报告了稳定 NP 胶体在有机和水介质中的氧化动力学。研究发现,制备的 NPs 中 Fe3O4 的比例取决于它们的尺寸,但与通常的假设不同,单色电子能量损失光谱结果显示,非均一纳米晶体中的 Fe2+ 浓度是均匀的,没有证据表明存在具有 γ-Fe2O3 外层的核/壳结构。此外,我们还发现,典型的配体交换程序用于去除表面的油酸酯封盖,会导致纳米粒子部分氧化,这表明表面配体在阻碍氧化反应方面起着关键作用。为了阐明封端剂在氧化还原转化中的作用,我们证明油酸酯覆盖率越高的 NPs,氧化过程越明显减慢。然后,我们通过考虑表面反应性与氧化物内部阳离子扩散之间的耦合,对这些发现进行了定量解释。最后,我们证明了氧化过程对 NPs 磁性能及其在射频磁场下加热效率的显著影响。总之,我们的研究结果阐明了设计氧化铁基纳米材料的重要性,这种材料具有更高的化学稳定性和更强的物理性质控制能力,而这正是其成功应用的关键所在。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemistry of Materials
工程技术-材料科学:综合
CiteScore
14.10
自引率
5.80%
发文量
929
审稿时长
1.5 months
期刊介绍:
The journal Chemistry of Materials focuses on publishing original research at the intersection of materials science and chemistry. The studies published in the journal involve chemistry as a prominent component and explore topics such as the design, synthesis, characterization, processing, understanding, and application of functional or potentially functional materials. The journal covers various areas of interest, including inorganic and organic solid-state chemistry, nanomaterials, biomaterials, thin films and polymers, and composite/hybrid materials. The journal particularly seeks papers that highlight the creation or development of innovative materials with novel optical, electrical, magnetic, catalytic, or mechanical properties. It is essential that manuscripts on these topics have a primary focus on the chemistry of materials and represent a significant advancement compared to prior research. Before external reviews are sought, submitted manuscripts undergo a review process by a minimum of two editors to ensure their appropriateness for the journal and the presence of sufficient evidence of a significant advance that will be of broad interest to the materials chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


