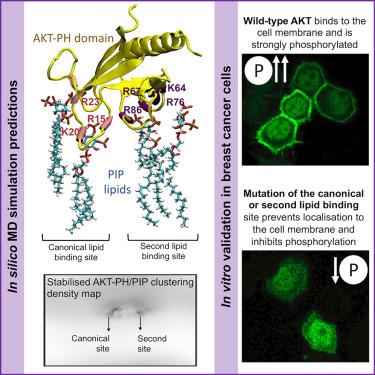
Two cooperative lipid binding sites within the pleckstrin homology domain are necessary for AKT binding and stabilization to the plasma membrane
IF 4.4
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Almost four decades after the identification of the AKT protein and understanding of its role in cancer, barriers remain in the translation of AKT inhibitors for clinical applications. Here, we provide new molecular insight into the first step of AKT activation where AKT binds to the plasma membrane and its orientation is stabilized in a bilayer with lateral heterogeneity (Lo-Ld phase coexistence). We have applied molecular dynamic simulations and molecular and cell biology approaches, and demonstrate that AKT recruitment to the membrane requires a second binding site in the AKT pleckstrin homology (PH) domain that acts cooperatively with the known canonical binding site. Given the precision with which we have identified the protein-lipid interactions, the study offers new directions for AKT-targeted therapy and for testing small molecules to target these specific amino acid-PIP molecular bonds.
pleckstrin同源结构域中的两个合作脂质结合位点是AKT与质膜结合和稳定的必要条件
在发现 AKT 蛋白并了解其在癌症中的作用近 40 年后,AKT 抑制剂的临床应用仍面临障碍。在这里,我们对 AKT 激活的第一步提供了新的分子见解,在这一步中,AKT 与质膜结合,其方向稳定在具有横向异质性(Lo-Ld 相共存)的双分子层中。我们应用分子动态模拟以及分子和细胞生物学方法证明,AKT 招募到膜上需要 AKT pleckstrin homology(PH)结构域中的第二个结合位点,它与已知的典型结合位点协同作用。鉴于我们精确地确定了蛋白质与脂质的相互作用,这项研究为 AKT 靶向治疗和测试针对这些特定氨基酸-PIP 分子键的小分子药物提供了新的方向。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Structure
生物-生化与分子生物学
CiteScore
8.90
自引率
1.80%
发文量
155
审稿时长
3-8 weeks
期刊介绍:
Structure aims to publish papers of exceptional interest in the field of structural biology. The journal strives to be essential reading for structural biologists, as well as biologists and biochemists that are interested in macromolecular structure and function. Structure strongly encourages the submission of manuscripts that present structural and molecular insights into biological function and mechanism. Other reports that address fundamental questions in structural biology, such as structure-based examinations of protein evolution, folding, and/or design, will also be considered. We will consider the application of any method, experimental or computational, at high or low resolution, to conduct structural investigations, as long as the method is appropriate for the biological, functional, and mechanistic question(s) being addressed. Likewise, reports describing single-molecule analysis of biological mechanisms are welcome.
In general, the editors encourage submission of experimental structural studies that are enriched by an analysis of structure-activity relationships and will not consider studies that solely report structural information unless the structure or analysis is of exceptional and broad interest. Studies reporting only homology models, de novo models, or molecular dynamics simulations are also discouraged unless the models are informed by or validated by novel experimental data; rationalization of a large body of existing experimental evidence and making testable predictions based on a model or simulation is often not considered sufficient.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


