Structure-Based Design of Covalent SARS-CoV-2 Papain-like Protease Inhibitors
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
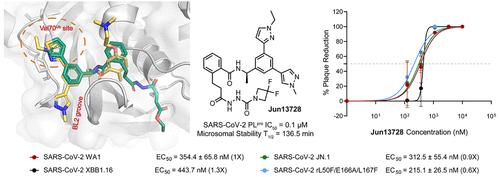
The COVID-19 pandemic is caused by SARS-CoV-2, a highly transmissible and pathogenic RNA betacoronavirus. Like other RNA viruses, SARS-CoV-2 continues to evolve with or without drug selection pressure, and many variants have emerged since the beginning of the pandemic. The papain-like protease, PLpro, is a cysteine protease that cleaves viral polyproteins as well as ubiquitin and ISG15 modifications from host proteins. Leveraging our recently discovered Val70Ub binding site in PLpro, we designed covalent PLpro inhibitors by connecting cysteine reactive warheads to the biarylphenyl PLpro inhibitors via flexible linkers. Several leads displayed potent enzymatic inhibition (IC50 = 0.1–0.3 μM) and antiviral activity (EC50 = 0.09–0.96 μM). Fumaramide inhibitors Jun13567 (15), Jun13728 (16), and Jun13714 (18) showed favorable in vivo pharmacokinetic properties with intraperitoneal injection. The X-ray crystal structure of PLpro with Jun13567 (15) validated our design strategy, revealing covalent conjugation between the catalytic Cys111 and the fumaramide warhead. The results suggest these covalent PLpro inhibitors are promising SARS-CoV-2 antiviral drug candidates.
基于结构设计的共价 SARS-CoV-2 Papain 类蛋白酶抑制剂
COVID-19 大流行是由 SARS-CoV-2 引起的,SARS-CoV-2 是一种高传播性和致病性的 RNA betacoronavirus。与其他 RNA 病毒一样,SARS-CoV-2 也在药物选择压力或无药物选择压力的情况下不断进化,自大流行开始以来已出现了许多变种。类似木瓜蛋白酶的 PLpro 是一种半胱氨酸蛋白酶,能裂解病毒多聚蛋白以及宿主蛋白中的泛素和 ISG15 修饰物。利用最近在 PLpro 中发现的 Val70Ub 结合位点,我们设计了共价 PLpro 抑制剂,通过灵活的连接体将半胱氨酸反应弹头连接到双芳基苯基 PLpro 抑制剂上。几种先导化合物显示了强效的酶抑制作用(IC50 = 0.1-0.3 μM)和抗病毒活性(EC50 = 0.09-0.96 μM)。富马酰胺抑制剂 Jun13567 (15)、Jun13728 (16) 和 Jun13714 (18) 通过腹腔注射显示出良好的体内药代动力学特性。PLpro与Jun13567(15)的X射线晶体结构验证了我们的设计策略,揭示了催化Cys111与富马酰胺弹头之间的共价连接。结果表明,这些共价 PLpro 抑制剂是很有前途的 SARS-CoV-2 抗病毒候选药物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


