Cell type mapping reveals tissue niches and interactions in subcortical multiple sclerosis lesions
IF 21.2
1区 医学
Q1 NEUROSCIENCES
引用次数: 0
Abstract
Multiple sclerosis (MS) is a chronic inflammatory disease of the central nervous system. Inflammation is gradually compartmentalized and restricted to specific tissue niches such as the lesion rim. However, the precise cell type composition of such niches, their interactions and changes between chronic active and inactive stages are incompletely understood. We used single-nucleus and spatial transcriptomics from subcortical MS and corresponding control tissues to map cell types and associated pathways to lesion and nonlesion areas. We identified niches such as perivascular spaces, the inflamed lesion rim or the lesion core that are associated with the glial scar and a cilia-forming astrocyte subtype. Focusing on the inflamed rim of chronic active lesions, we uncovered cell–cell communication events between myeloid, endothelial and glial cell types. Our results provide insight into the cellular composition, multicellular programs and intercellular communication in tissue niches along the conversion from a homeostatic to a dysfunctional state underlying lesion progression in MS. Lerma-Martin et al. generated a paired single-nucleus RNA sequencing and spatial transcriptomics dataset from subcortical multiple sclerosis lesions, identifying spatial niches and key cell interactions driving inflammation and disease progression at the lesion rim.
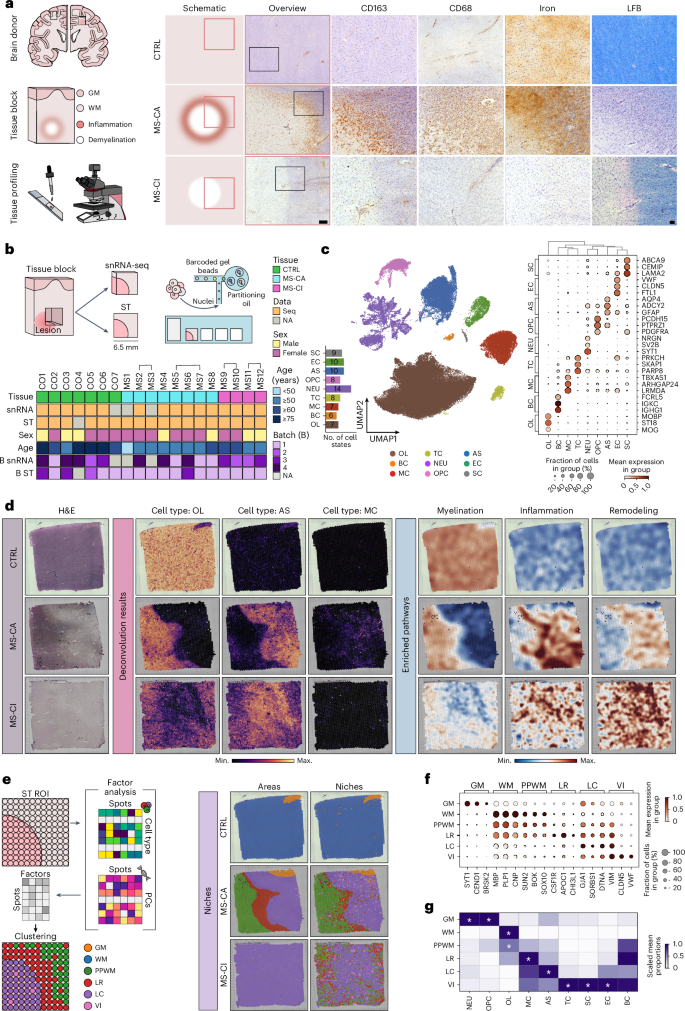
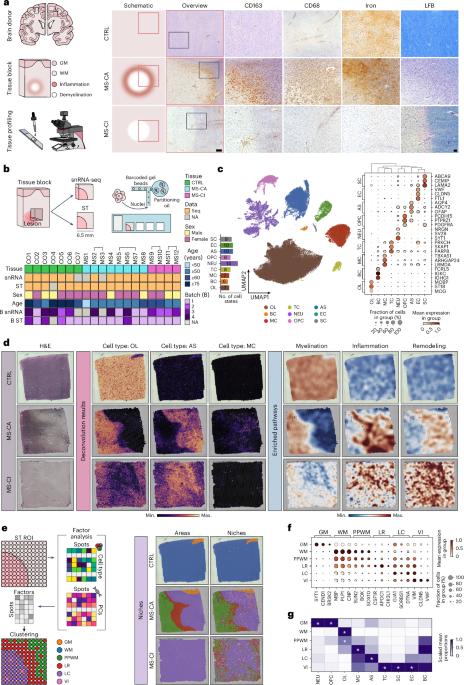
细胞类型图揭示皮层下多发性硬化病变中的组织龛位和相互作用
多发性硬化症(MS)是一种中枢神经系统慢性炎症性疾病。炎症逐渐分化并局限于特定的组织龛,如病变边缘。然而,人们对这些龛位的精确细胞类型组成、它们之间的相互作用以及慢性活动期和非活动期之间的变化还不完全了解。我们利用皮层下多发性硬化症和相应对照组织的单核和空间转录组学来绘制病变和非病变区域的细胞类型和相关通路。我们确定了与胶质瘢痕和纤毛形成星形胶质细胞亚型相关的壁龛,如血管周围空间、炎性病变边缘或病变核心。我们重点研究了慢性活动性病变的炎症边缘,发现了骨髓细胞、内皮细胞和胶质细胞类型之间的细胞-细胞通讯事件。我们的研究结果让我们深入了解了多发性硬化症病变从平衡状态向功能障碍状态转化过程中组织龛位中的细胞组成、多细胞程序和细胞间通讯。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature neuroscience
医学-神经科学
CiteScore
38.60
自引率
1.20%
发文量
212
审稿时长
1 months
期刊介绍:
Nature Neuroscience, a multidisciplinary journal, publishes papers of the utmost quality and significance across all realms of neuroscience. The editors welcome contributions spanning molecular, cellular, systems, and cognitive neuroscience, along with psychophysics, computational modeling, and nervous system disorders. While no area is off-limits, studies offering fundamental insights into nervous system function receive priority.
The journal offers high visibility to both readers and authors, fostering interdisciplinary communication and accessibility to a broad audience. It maintains high standards of copy editing and production, rigorous peer review, rapid publication, and operates independently from academic societies and other vested interests.
In addition to primary research, Nature Neuroscience features news and views, reviews, editorials, commentaries, perspectives, book reviews, and correspondence, aiming to serve as the voice of the global neuroscience community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


