“Balloon-like” biomimetic erythrocyte vesicles potentiate photodynamic therapy for inducing immunogenic cell death
IF 13.2
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
Photodynamic therapy (PDT) for cancer is known for its minimal invasiveness and safe characteristics, but the hypoxic tumor microenvironment still limits efficacy. Herein, we have developed a novel “balloon-like” biomimetic erythrocyte vesicle, which is constructed by loading chlorin e6 (Ce6) and oxygenated hemoglobin (Hb) in a folate (FA)-modified, PEGylated liposome, denoted as Ce6-Hb-FA@lip (CHFL). The CHFL exhibits high biocompatibility and good stability in PBS solution. Moreover, CHFL possesses the “balloon-like” elastic structure for oxygen binding with the change of volume. Both in vitro and in vivo studies demonstrate an effective PDT of CHFL, which significantly reverses the hypoxic microenvironment of tumor cells through the targeted cotransmission of Ce6 and Hb. Additionally, this biomimetic erythrocyte vesicle can induce oxidative stress to enhance tumor immunogenicity and trigger immunogenic cell death (ICD). Thus, the dying tumor cells can activate both dendritic cells (DCs) and T lymphocytes, which, in turn, initiates the antitumor immune response by releasing the damage-associated molecular patterns (DAMPs). This effective and safe platform holds great promise in the development of nanomedicines for innovative oxygen-enhanced PDT in the treatment of cancer.
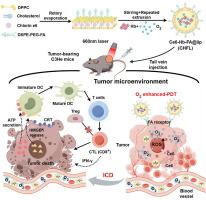
"气球状 "仿生红细胞囊泡可增强光动力疗法诱导免疫性细胞死亡的效果
癌症光动力疗法(PDT)以其微创性和安全性著称,但缺氧的肿瘤微环境仍限制了其疗效。在此,我们开发了一种新型的 "气球状 "仿生红细胞囊泡,它是通过在叶酸(FA)修饰的 PEG 化脂质体(称为 Ce6-Hb-FA@lip (CHFL))中加入氯素 e6 (Ce6) 和氧合血红蛋白(Hb)而构建的。CHFL 在 PBS 溶液中具有很高的生物相容性和良好的稳定性。此外,CHFL 还具有 "气球状 "弹性结构,可随着体积的变化与氧气结合。体外和体内研究都表明,CHFL 能有效地进行光导放疗,通过 Ce6 和 Hb 的靶向共传递,显著逆转肿瘤细胞的缺氧微环境。此外,这种仿生红细胞囊泡还能诱导氧化应激,增强肿瘤免疫原性,引发免疫原性细胞死亡(ICD)。因此,死亡的肿瘤细胞可以激活树突状细胞(DCs)和 T 淋巴细胞,进而通过释放损伤相关分子模式(DAMPs)启动抗肿瘤免疫反应。这种有效而安全的平台为开发用于治疗癌症的创新性氧增强光动力疗法的纳米药物带来了巨大希望。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


