Oxidation and degradation of nitrobenzene by ozone micro-nano bubbles
IF 4.1
2区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
The remediation of ozone oxidation to nitrobenzene (NB) contaminated groundwater has limitations such as low ozone solubility and poor gas transmission capacity. In this paper, we combine the ozone oxidation and the micro-nano bubbles (MNBs) and investigate the oxidation of the degradation of NB using a semi-continuous experimental approach. The experimental results show that the degradation efficiency of NB by ozone MNBs system (98.69%) is significantly higher than that of traditional ozone oxidation. The presence of MNBs significantly increase the rate of ·OH, ·O2– and 1O2 production compared to large bubbles produced by ozone aeration. This is due to OH– adsorbed at the interface will accelerate the conversion of ozone to ·OH during bubble rupture. And conversion between O3,·OH, ·O2– and 1O2。 The degradation of NB in ozone MNBs could be described by quasi-first-order kinetics. The degradation kinetics of ozone MNBs are obviously affected by the initial concentration of NB, the initial pH of the reaction system, salinity, gas species and flow rate. Oxidative degradation products (p-benzoquinone, phenol, o-nitrophenol, p-nitrophenol, aniline, acetic acid, NO3–, etc.) have been identified by GC–MS, IC, UV–Vis. They show that the degradation results from the attack of the aromatic ring and –NO2 of NB through nitrification, free radicals and O3. This research introduces a novel approach for degrading NB and offers crucial insights into the degradation of organic substances using MNBs.
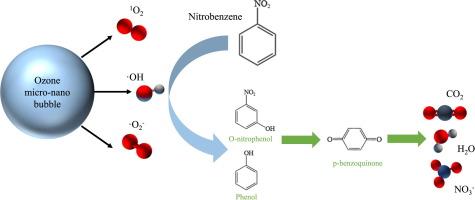
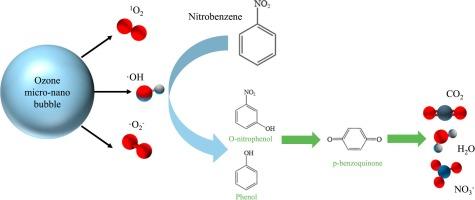
臭氧微纳气泡对硝基苯的氧化和降解作用
臭氧氧化法修复受硝基苯(NB)污染的地下水存在臭氧溶解度低、气体传输能力差等局限性。本文将臭氧氧化与微纳米气泡(MNBs)相结合,采用半连续实验方法研究了氧化降解 NB 的方法。实验结果表明,臭氧 MNBs 系统对 NB 的降解效率(98.69%)明显高于传统的臭氧氧化法。与臭氧曝气产生的大气泡相比,MNB 的存在大大提高了 -OH、-O2- 和 1O2 的产生速率。这是由于吸附在界面上的 OH- 会在气泡破裂时加速臭氧向 -OH 的转化。臭氧 MNB 中 NB 的降解可以用准一阶动力学来描述。臭氧 MNB 的降解动力学明显受到 NB 初始浓度、反应体系初始 pH 值、盐度、气体种类和流速的影响。通过 GC-MS、IC 和 UV-Vis 对氧化降解产物(对苯醌、苯酚、邻硝基苯酚、对硝基苯酚、苯胺、乙酸、NO3- 等)进行了鉴定。研究结果表明,降解是通过硝化、自由基和 O3 对 NB 的芳香环和 -NO2 进行攻击的结果。这项研究介绍了一种降解 NB 的新方法,并为利用 MNB 降解有机物提供了重要的启示。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Science
工程技术-工程:化工
CiteScore
7.50
自引率
8.50%
发文量
1025
审稿时长
50 days
期刊介绍:
Chemical engineering enables the transformation of natural resources and energy into useful products for society. It draws on and applies natural sciences, mathematics and economics, and has developed fundamental engineering science that underpins the discipline.
Chemical Engineering Science (CES) has been publishing papers on the fundamentals of chemical engineering since 1951. CES is the platform where the most significant advances in the discipline have ever since been published. Chemical Engineering Science has accompanied and sustained chemical engineering through its development into the vibrant and broad scientific discipline it is today.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


