Spatiotemporal protein interactome profiling through condensation-enhanced photocrosslinking
IF 19.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Resolving protein–protein interactions (PPIs) inside biomolecular condensates is crucial for elucidating their functions and regulation mechanisms. The transient nature of condensates and the multiple localizations of clients, however, have rendered it challenging to determine compartment-specific PPIs. Here we developed a condensation-enhanced, spatially directed, metabolic incorporation-assisted photocrosslinking strategy—termed DenseMAP—for spatiotemporally resolved dissection of the direct protein interactome within condensates. By leveraging our condensation-enhanced photocrosslinker and the spatially directed biotin tagging, DenseMAP enabled stress-granule-specific interactome mapping of the N6-methyladenosine readers YTHDF1 and YTHDF2, and uncovered the functional role of phosphorylation on the SARS-CoV-2 nucleocapsid protein in regulating virus replication. Further applying DenseMAP for direct interactome mapping of the subcompartmental scaffold protein NPM1 deciphered nucleolar granular component proteome, and unveiled the critical role of SUMOylation in controlling nucleolar proteome homeostasis. DenseMAP provides a platform technology for analysing functional PPI networks within subcellular and subcompartmental condensates under diverse physiological and/or pathological settings. Now a generalizable method that couples spatially directed tagging with condensation-enhanced crosslinking—termed DenseMAP—has been developed for profiling the spatiotemporal protein interactome within biomolecular condensates. By linking the information between protein interactions and subcellular locations, DenseMAP has uncovered regulatory mechanisms in subcellular and subcompartmental condensates.
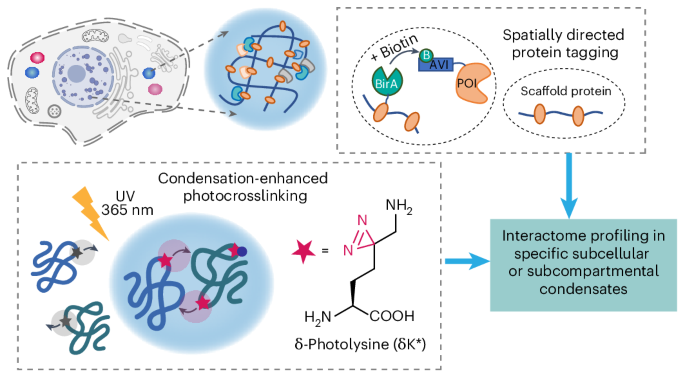

通过缩聚增强光交联技术绘制时空蛋白质相互作用组图谱
解析生物分子凝聚体内的蛋白质-蛋白质相互作用(PPIs)对于阐明其功能和调控机制至关重要。然而,凝结物的瞬时性和客户的多重定位使得确定特异性 PPIs 具有挑战性。在这里,我们开发了一种凝集增强、空间定向、代谢掺入辅助的光交联策略--DenseMAP--用于对凝集体内的直接蛋白质相互作用组进行时空分辨分析。通过利用我们的凝集增强型光交联剂和空间定向生物素标记,DenseMAP实现了N6-甲基腺苷阅读器YTHDF1和YTHDF2的应激颗粒特异性相互作用组图谱,并揭示了SARS-CoV-2核壳蛋白上的磷酸化在调控病毒复制中的功能作用。进一步应用 DenseMAP 直接绘制亚室支架蛋白 NPM1 的相互作用组图谱,破译了核小体颗粒成分蛋白质组,揭示了 SUMOylation 在控制核小体蛋白质组平衡中的关键作用。DenseMAP 为分析亚细胞和亚室凝聚物在不同生理和/或病理环境下的功能性 PPI 网络提供了一种平台技术。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature chemistry
化学-化学综合
CiteScore
29.60
自引率
1.40%
发文量
226
审稿时长
1.7 months
期刊介绍:
Nature Chemistry is a monthly journal that publishes groundbreaking and significant research in all areas of chemistry. It covers traditional subjects such as analytical, inorganic, organic, and physical chemistry, as well as a wide range of other topics including catalysis, computational and theoretical chemistry, and environmental chemistry.
The journal also features interdisciplinary research at the interface of chemistry with biology, materials science, nanotechnology, and physics. Manuscripts detailing such multidisciplinary work are encouraged, as long as the central theme pertains to chemistry.
Aside from primary research, Nature Chemistry publishes review articles, news and views, research highlights from other journals, commentaries, book reviews, correspondence, and analysis of the broader chemical landscape. It also addresses crucial issues related to education, funding, policy, intellectual property, and the societal impact of chemistry.
Nature Chemistry is dedicated to ensuring the highest standards of original research through a fair and rigorous review process. It offers authors maximum visibility for their papers, access to a broad readership, exceptional copy editing and production standards, rapid publication, and independence from academic societies and other vested interests.
Overall, Nature Chemistry aims to be the authoritative voice of the global chemical community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


