Akt-FoxO signaling drives co-adaptation to insecticide and host plant stresses in an herbivorous insect
IF 13
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
Introduction
Ongoing interactions between host and herbivorous insect trigger a co-evolutionary arms race. Genetic diversity within insects facilitates their adaptation to phytochemicals and their derivatives, including plant-derived insecticides. Cytochrome P450s play important roles in metabolizing phytochemicals and insecticides, due to their diversity and almost perfect evolution.
Objectives
This study aims to uncover a common molecular mechanism in herbivorous insects by investigating the role of kinase-transcription factor regulation of P450s in conferring tolerance to both insecticides and phytochemicals.
Methods
RNA interference, transcriptome sequencing, insecticide, and phytochemical bioassays were conducted to validate the functions of Akt, FoxO, and candidate P450s. Dual-luciferase activity assays were employed to identify the regulation of P450s by the Akt-FoxO signaling pathway. Recombinant P450 enzymes were utilized to investigate the metabolism of insecticides and phytochemicals.
Results
We identified an Akt-FoxO signaling cascade, a representative of kinase-transcription factor pathways. This cascade mediates the expression of eight P450 enzymes involved in the metabolism of insecticides and phytochemicals in Nilaparvata lugens. These P450s are from different families and with different substrate selectivity, enabling them to respectively metabolize insecticides and phytochemicals with structure diversity. Nevertheless, the eight P450 genes were up-regulated by FoxO, which was inhibited in a higher cascade by Akt through phosphorylation. The discovery of the Akt-FoxO signaling pathway regulating a series of P450 genes elucidates the finely tuned regulatory mechanism in insects for adapting to phytochemicals and insecticides.
Conclusion
These finding sheds light on the physiological balance maintained by these regulatory processes. The work provides the experimental evidence of co-adaptation to the stresses imposed by host plant and insecticide within the model of the kinase-TF involving various P450s. This model provides a comprehensive view of how pests adapt to multiple environmental stresses.
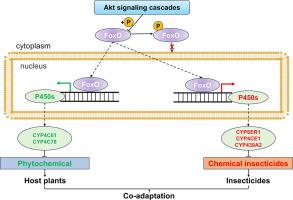
Akt-FoxO信号驱动一种食草昆虫共同适应杀虫剂和寄主植物胁迫
引言 宿主与食草昆虫之间持续不断的相互作用引发了一场共同进化的军备竞赛。昆虫体内的遗传多样性促进了它们对植物化学物质及其衍生物(包括植物杀虫剂)的适应。本研究旨在通过研究激酶-转录因子调控 P450s 在赋予昆虫对杀虫剂和植物化学物质耐受性方面的作用,揭示食草昆虫的共同分子机制。方法通过RNA干扰、转录组测序、杀虫剂和植物化学物质生物测定来验证Akt、FoxO和候选P450s的功能。采用双荧光素酶活性测定来确定 Akt-FoxO 信号通路对 P450s 的调控。利用重组 P450 酶来研究杀虫剂和植物化学物质的代谢。该级联介导了 Nilaparvata lugens 中参与杀虫剂和植物化学物质代谢的 8 种 P450 酶的表达。这些 P450 来自不同的家族,具有不同的底物选择性,使它们能够分别代谢具有结构多样性的杀虫剂和植物化学物质。然而,这八个 P450 基因都受到 FoxO 的上调,而 FoxO 又受到 Akt 的磷酸化抑制。Akt-FoxO 信号通路调控一系列 P450 基因的发现,阐明了昆虫适应植物化学物质和杀虫剂的微调调控机制。这项工作提供了实验证据,证明在涉及各种 P450 的激酶-TF 模型中,寄主植物和杀虫剂施加的压力具有共同适应性。该模型为害虫如何适应多种环境胁迫提供了一个全面的视角。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


