Tetrahydrocurcumin targets TRIP13 inhibiting the interaction of TRIP13/USP7/c-FLIP to mediate c-FLIP ubiquitination in triple-negative breast cancer
IF 11.4
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
Introduction
Triple-negative breast cancer (TNBC) has a high mortality rate and limited treatment options. Tetrahydrocurcumin (THC), a major metabolite of curcumin, has potential antitumor activities. However, the antitumor effects and mechanism of THC in TNBC remain elusive.Objectives
To investigate the mechanism of THC in combating TNBC by targeting TRIP13 to disrupt the interaction of the TRIP13/USP7/c-FLIP complex and mediate c-FLIP ubiquitination both in vitro and in vivo.Methods
Apoptosis was measured by TUNEL and flow cytometry. Click chemistry-based target fishing, CETSA, DARTS, and SPR were used to identify direct target of THC. Protein interactions was examined using co-immunoprecipitation. The role of USP7 in THC-mediated c-FLIP ubiquitination was evaluated by in vitro deubiquitination assay. Human breast cancer clinical samples were employed to assess the expression of c-FLIP, TRIP13, and USP7. The impact of THC on USP7/TRIP13/c-FLIP was analyzed using co-immunoprecipitation, confocal microscopy, molecular docking and dynamics simulations.Results
THC effectively inhibits TNBC cell proliferation and tumor growth in vitro and in vivo without significant toxicity. Mechanistically, THC induces extrinsic apoptosis in TNBC primarily by promoting degradation of c-FLIP, a key negative regulator in the apoptotic pathway. Furthermore, utilizing click chemistry-based target fishing, we identified TRIP13, a component of the highly conserved AAA ATPase family, as a direct target of THC in combating TNBC. Interestingly, contrary to previous drug-target studies, the knockdown of TRIP13 further amplified the antitumor effects of THC. After in-depth investigation, it was revealed that TRIP13 forms a trimeric complex with USP7 and c-FLIP in TNBC cells. THC specifically targets TRIP13 to disrupt the interaction of TRIP13/USP7/c-FLIP, leading to the ubiquitination of c-FLIP, ultimately inducing extrinsic apoptosis.Conclusions
These findings offer new insights into the novel molecular mechanisms of anti-TNBC effects of THC and present a promising targeted therapeutic strategy for TNBC.
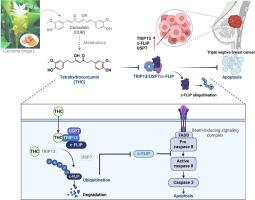
四氢姜黄素靶向 TRIP13,抑制 TRIP13/USP7/c-FLIP 的相互作用,从而介导三阴性乳腺癌中 c-FLIP 的泛素化
导言三阴性乳腺癌(TNBC)死亡率高,治疗方案有限。四氢姜黄素(THC)是姜黄素的一种主要代谢产物,具有潜在的抗肿瘤活性。目的通过靶向TRIP13来破坏TRIP13/USP7/c-FLIP复合物的相互作用,并在体外和体内介导c-FLIP泛素化,从而研究THC抗击TNBC的机制。采用基于点击化学的靶标捕获、CETSA、DARTS 和 SPR 方法确定 THC 的直接靶标。使用共免疫沉淀法检测了蛋白质之间的相互作用。体外去泛素化试验评估了 USP7 在 THC 介导的 c-FLIP 泛素化中的作用。采用人类乳腺癌临床样本来评估 c-FLIP、TRIP13 和 USP7 的表达。结果四氢大麻酚在体外和体内均能有效抑制 TNBC 细胞增殖和肿瘤生长,且无明显毒性。从机理上讲,四氢大麻酚主要通过促进 c-FLIP 的降解来诱导 TNBC 细胞的外源性凋亡,c-FLIP 是凋亡途径中的一个关键负调控因子。此外,通过基于点击化学的靶点捕获,我们发现TRIP13(高度保守的AAA ATPase家族的一个成员)是THC抗击TNBC的直接靶点。有趣的是,与以往的药物靶点研究相反,TRIP13的敲除进一步增强了THC的抗肿瘤作用。经过深入研究发现,TRIP13在TNBC细胞中与USP7和c-FLIP形成三聚体复合物。THC特异性靶向TRIP13,破坏TRIP13/USP7/c-FLIP的相互作用,导致c-FLIP泛素化,最终诱导细胞外源性凋亡。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


