Structure-selectivity relationship of anion exchange resins with different quaternary amine functional groups for highly selective removal of PFAS from chromium-plating wastewater
IF 11.4
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
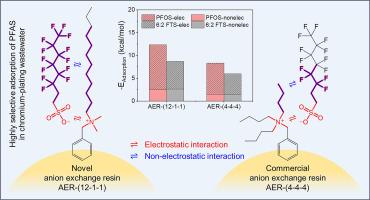
Anion exchange resin (AER) adsorption is an effective technology for the removal of per- and polyfluoroalkyl substances (PFAS) from wastewater. However, existing AERs with tributylamine functional groups have poor adsorption selectivity for perfluorinated carboxylic acids (PFCAs) and 6:2 fluorotelomer sulfonate (6:2 FTS), and the structure-selectivity relationship is still unclear. In this study, several novel gel AERs with long-chain amine groups were prepared. It was found that their adsorption selectivity for 6:2 FTS was 3.3–5.1 times that of commercial AERs, and the adsorption amount for 6:2 FTS in chromium-plating wastewater was 2.1 times that of the commercial PFA694E. On this basis, we synthesized 16 AERs with different quaternary amine functional groups, and explored the structure-selectivity relationship through the selectivity coefficients and adsorption energies of different AERs for seven typical PFAS. The order of adsorption selectivity of AERs with different quaternary amine groups for PFAS was AER-(12–1-1) > AER-(8–1-1) > AER-(4–4-4) > AER-(4–1-1) ≈ AER-(2–2-2) > AER-(1–1-1), where the three numbers are the carbon-chain lengths of the three alkyl groups attached to the nitrogen atom of the quaternary amine group. Density functional theory (DFT) calculations confirmed the enhanced adsorption selectivity and contribution of both non-electrostatic and electrostatic interactions by the long-chain amine groups, and a quantitative relationship between theoretical calculations and experimental results was established. These results could provide guidance for the development of efficient adsorbents for PFAS removal from wastewater.
具有不同季胺官能团的阴离子交换树脂在高选择性去除镀铬废水中 PFAS 方面的结构-选择性关系
阴离子交换树脂(AER)吸附技术是去除废水中全氟和多氟烷基物质(PFAS)的有效技术。然而,现有的具有三丁胺官能团的阴离子交换树脂对全氟羧酸(PFCAs)和 6:2 氟橡胶磺酸盐(6:2 FTS)的吸附选择性较差,其结构与选择性之间的关系尚不清楚。本研究制备了几种带有长链胺基团的新型凝胶 AER。研究发现,它们对 6:2 FTS 的吸附选择性是商用 AER 的 3.3-5.1 倍,对镀铬废水中 6:2 FTS 的吸附量是商用 PFA694E 的 2.1 倍。在此基础上,我们合成了 16 种具有不同季胺官能团的 AER,并通过不同 AER 对 7 种典型 PFAS 的选择性系数和吸附能来探讨其结构-选择性关系。不同季胺基团的 AER 对 PFAS 的吸附选择性顺序为 AER-(12-1-1) > AER-(8-1-1) > AER-(4-4-4) > AER-(4-1-1) ≈ AER-(2-2-2) > AER-(1-1-1)。密度泛函理论(DFT)计算证实了长链胺基团增强的吸附选择性以及非静电和静电相互作用的贡献,并建立了理论计算与实验结果之间的定量关系。这些结果可为开发去除废水中 PFAS 的高效吸附剂提供指导。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Water Research
环境科学-工程:环境
CiteScore
20.80
自引率
9.40%
发文量
1307
审稿时长
38 days
期刊介绍:
Water Research, along with its open access companion journal Water Research X, serves as a platform for publishing original research papers covering various aspects of the science and technology related to the anthropogenic water cycle, water quality, and its management worldwide. The audience targeted by the journal comprises biologists, chemical engineers, chemists, civil engineers, environmental engineers, limnologists, and microbiologists. The scope of the journal include:
•Treatment processes for water and wastewaters (municipal, agricultural, industrial, and on-site treatment), including resource recovery and residuals management;
•Urban hydrology including sewer systems, stormwater management, and green infrastructure;
•Drinking water treatment and distribution;
•Potable and non-potable water reuse;
•Sanitation, public health, and risk assessment;
•Anaerobic digestion, solid and hazardous waste management, including source characterization and the effects and control of leachates and gaseous emissions;
•Contaminants (chemical, microbial, anthropogenic particles such as nanoparticles or microplastics) and related water quality sensing, monitoring, fate, and assessment;
•Anthropogenic impacts on inland, tidal, coastal and urban waters, focusing on surface and ground waters, and point and non-point sources of pollution;
•Environmental restoration, linked to surface water, groundwater and groundwater remediation;
•Analysis of the interfaces between sediments and water, and between water and atmosphere, focusing specifically on anthropogenic impacts;
•Mathematical modelling, systems analysis, machine learning, and beneficial use of big data related to the anthropogenic water cycle;
•Socio-economic, policy, and regulations studies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


