Reconsideration of the role of hydrogen peroxide in peroxymonocarbonate-based oxidation system for pollutant control
IF 11.4
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
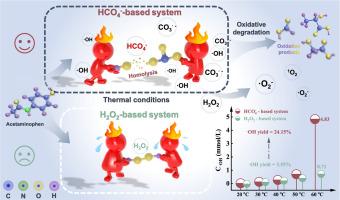
Advanced oxidation processes that utilize peroxymonocarbonate (HCO4-), generated in-situ through the reaction of HCO3- and H2O2, are employed for the removal of pollutants in water. Nevertheless, the precise role of H2O2 in these processes remains a subject of debate. This study established a HCO4--based oxidation system using NaHCO3 and H2O2 for the degradation of acetaminophen and investigated the activation mechanisms of coexisting oxidants. Under thermal activation conditions, the O![]() O bond in HCO4- (HO
O bond in HCO4- (HO![]() OCOO-) was more readily cleaved than the O
OCOO-) was more readily cleaved than the O![]() O bond in the co-existing oxidant H2O2 (HO
O bond in the co-existing oxidant H2O2 (HO![]() OH), leading to the generation of reactive oxygen species (ROS). Based on kinetics and ROS evaluation, H2O2 primarily served to form HCO4- rather than converting to ·OH or O2, with HCO4- acting as the primary oxidant for degradation through the formation of and ·OH. In this oxidation system, H2O2 utilization efficiency for ·OH production reached 27.34 %, ·OH yield reached 24.15 % and acetaminophen degradation efficiency realized 83 % at 60 °C with 20 mM HCO3- and 20 mM H2O2. The apparent activation energy of acetaminophen degradation and HCO4- activation were calculated as 90.83 kJ mol-1 and 18.81 kJ mol-1, respectively. Moreover, a novel CO2-derived HCO4--based system led to a comparable acetaminophen degradation efficiency of 82 % and a higher kobs of 0.028 min-1. The system optimization and ROS evaluation suggest that high concentration of H2O2 inhibited the degradation and quenched and ·OH to yield ·O2- and 1O2. Furthermore, EPR analysis and quenching experiments indicate that was mainly responsible for acetaminophen degradation. This work provides fundamental understanding of the HCO4--based oxidation system.
OH), leading to the generation of reactive oxygen species (ROS). Based on kinetics and ROS evaluation, H2O2 primarily served to form HCO4- rather than converting to ·OH or O2, with HCO4- acting as the primary oxidant for degradation through the formation of and ·OH. In this oxidation system, H2O2 utilization efficiency for ·OH production reached 27.34 %, ·OH yield reached 24.15 % and acetaminophen degradation efficiency realized 83 % at 60 °C with 20 mM HCO3- and 20 mM H2O2. The apparent activation energy of acetaminophen degradation and HCO4- activation were calculated as 90.83 kJ mol-1 and 18.81 kJ mol-1, respectively. Moreover, a novel CO2-derived HCO4--based system led to a comparable acetaminophen degradation efficiency of 82 % and a higher kobs of 0.028 min-1. The system optimization and ROS evaluation suggest that high concentration of H2O2 inhibited the degradation and quenched and ·OH to yield ·O2- and 1O2. Furthermore, EPR analysis and quenching experiments indicate that was mainly responsible for acetaminophen degradation. This work provides fundamental understanding of the HCO4--based oxidation system.
重新考虑过氧化氢在基于过氧一碳酸盐的污染物控制氧化系统中的作用
利用 HCO3- 和 H2O2 反应生成的过氧化单碳酸盐(HCO4-)的高级氧化工艺,被用于去除水中的污染物。然而,H2O2 在这些过程中的确切作用仍是一个争论的话题。本研究利用 NaHCO3 和 H2O2 建立了一个基于 HCO4 的氧化系统来降解对乙酰氨基酚,并研究了共存氧化剂的活化机制。在热激活条件下,HCO4-(HO-OCOO-)中的O-O键比共存氧化剂H2O2(HO-OH)中的O-O键更容易裂解,从而产生活性氧(ROS)。根据动力学和 ROS 评估,H2O2 主要用于形成 HCO4-,而不是转化为 -OH 或 O2,HCO4- 通过形成 CO3-- 和 -OH 成为降解的主要氧化剂。在该氧化体系中,20 mM HCO3- 和 20 mM H2O2 在 60 °C 条件下,产生 -OH 的 H2O2 利用率达到 27.34%,-OH 产量达到 24.15%,对乙酰氨基酚的降解效率达到 83%。计算得出的对乙酰氨基酚降解表观活化能和 HCO4- 活化表观活化能分别为 90.83 kJ mol-1 和 18.81 kJ mol-1。此外,基于 CO2 衍生 HCO4- 的新型系统的对乙酰氨基酚降解效率为 82%,Kobs 为 0.028 min-1。系统优化和 ROS 评估表明,高浓度 H2O2 可抑制降解,并淬灭 CO3-- 和 -OH 生成 -O2- 和 1O2。此外,EPR 分析和淬灭实验表明 CO3--是对乙酰氨基酚降解的主要原因。这项研究为了解基于 HCO4 的氧化系统提供了基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Water Research
环境科学-工程:环境
CiteScore
20.80
自引率
9.40%
发文量
1307
审稿时长
38 days
期刊介绍:
Water Research, along with its open access companion journal Water Research X, serves as a platform for publishing original research papers covering various aspects of the science and technology related to the anthropogenic water cycle, water quality, and its management worldwide. The audience targeted by the journal comprises biologists, chemical engineers, chemists, civil engineers, environmental engineers, limnologists, and microbiologists. The scope of the journal include:
•Treatment processes for water and wastewaters (municipal, agricultural, industrial, and on-site treatment), including resource recovery and residuals management;
•Urban hydrology including sewer systems, stormwater management, and green infrastructure;
•Drinking water treatment and distribution;
•Potable and non-potable water reuse;
•Sanitation, public health, and risk assessment;
•Anaerobic digestion, solid and hazardous waste management, including source characterization and the effects and control of leachates and gaseous emissions;
•Contaminants (chemical, microbial, anthropogenic particles such as nanoparticles or microplastics) and related water quality sensing, monitoring, fate, and assessment;
•Anthropogenic impacts on inland, tidal, coastal and urban waters, focusing on surface and ground waters, and point and non-point sources of pollution;
•Environmental restoration, linked to surface water, groundwater and groundwater remediation;
•Analysis of the interfaces between sediments and water, and between water and atmosphere, focusing specifically on anthropogenic impacts;
•Mathematical modelling, systems analysis, machine learning, and beneficial use of big data related to the anthropogenic water cycle;
•Socio-economic, policy, and regulations studies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


