Design and synthesis of 3,5-disubstituted isoxazoles by Cu-mediated 1,3-dipolar cycloaddition and their in silico evaluation as potential GABAB receptor modulators
IF 2.1
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
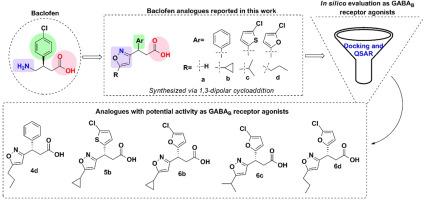
In this work, we report the synthesis of three series of 3,5-disubstituted isoxazoles (4a-d, 5a-d and 6a-d), as analogues of the clinically important drug baclofen. The isoxazole ring systems were assembled through the key copper-catalyzed 1,3-dipolar cycloaddition reaction between a nitrile oxide (generated in situ from nitro compounds) and an alkyne. An X-ray crystallography study shows that the 1,3-dipolar cycloaddition reactions proceeded with very high regioselectively, leading exclusively to the formation of the 3,5-disubstituted regio isomers. Additionally, it was possible to obtain these compounds in enantiomerically pure form using SuperQuat-type chiral auxiliary. As suggested by our QSAR analysis and docking studies, these analogues have the potential to be biologically active as GABAB receptor modulators. Finally, the absorption, distribution, properties of metabolism and excretion (ADME) of the synthesized molecules was also determined by a computational approach. From this work we obtained five molecules ((S)-4d, (S)-5b, (S)-6b, (S)-6c and (S)-6d) as potential GABAB receptor agonists.
通过铜介导的 1,3-二极环加成法设计和合成 3,5-二取代的异噁唑,并将其作为潜在的 GABAB 受体调节剂进行硅学评估
在这项工作中,我们报告了三个系列的 3,5-二取代异噁唑(4a-d、5a-d 和 6a-d)的合成,它们是临床上重要药物巴氯芬的类似物。这些异噁唑环系统是通过腈氧化物(由硝基化合物原位生成)和炔烃之间关键的铜催化 1,3-二极环加成反应组装而成的。X 射线晶体学研究表明,1,3-二极环加成反应具有极高的区域选择性,只能形成 3,5- 二取代的区域异构体。此外,利用 SuperQuat 型手性助剂还可以获得这些化合物的对映体纯度。根据我们的 QSAR 分析和对接研究,这些类似物具有作为 GABAB 受体调节剂的生物活性潜力。最后,我们还通过计算方法确定了合成分子的吸收、分布、代谢和排泄特性(ADME)。通过这项工作,我们获得了五个分子((S)-4d、(S)-5b、(S)-6b、(S)-6c 和 (S)-6d)作为潜在的 GABAB 受体激动剂。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Tetrahedron
化学-有机化学
CiteScore
3.90
自引率
4.80%
发文量
439
审稿时长
34 days
期刊介绍:
Tetrahedron publishes full accounts of research having outstanding significance in the broad field of organic chemistry and its related disciplines, such as organic materials and bio-organic chemistry.
Regular papers in Tetrahedron are expected to represent detailed accounts of an original study having substantially greater scope and details than that found in a communication, as published in Tetrahedron Letters.
Tetrahedron also publishes thematic collections of papers as special issues and ''Reports'', commissioned in-depth reviews providing a comprehensive overview of a research area.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


