Pd(II)-catalyzed C–H annulation and lactonization of indole-2-carboxamides with hydroxyalkynoates using air as an oxidant
引用次数: 0
Abstract
A novel palladium(II) catalyzed C–H annulation strategy has been developed for the synthesis of a diverse range of furo[3′,4':5,6]pyrido[3,4-b]indole-1,5(3H)-dione scaffolds. This is the first report on the construction of polycyclic carboline frameworks from indole-2-carboxamides and 4-hydroxy-2-alkynoates. This method provides a direct access to fused carbolines that are closely resemble to biologically active β-carboline frameworks.
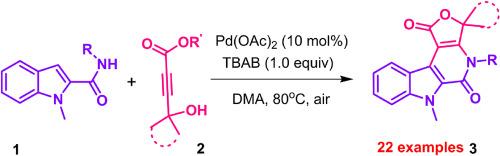
以空气为氧化剂催化吲哚-2-甲酰胺与羟基炔酸盐的 C-H 环化和内酯化反应
我们开发了一种新型钯(II)催化 C-H 环化策略,用于合成多种呋喃并[3′,4':5,6]吡啶并[3,4-b]吲哚-1,5(3H)-二酮支架。这是首次报道从吲哚-2-羧酰胺和 4-羟基-2-炔酸盐构建多环咔啉框架。这种方法提供了直接获得与具有生物活性的 β-咔啉框架非常相似的融合咔啉的途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


