Total synthesis of natural benzoazepinoquinolinone alkaloid irrepairzepine via Au(I)-catalyzed cycloisomerization and regioselective functionalization
IF 1.5
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Irrepairzepine (1), a unique benzoazepinoquinolinone alkaloid isolated from an endophytic fungus, exhibits synthetic lethality targeting in PTEN-deficient glioblastoma cells. Herein, the first synthesis of irrepairzepine was achieved through a series of high-yielding reactions. The key steps include the synthesis of quinoline via the Au(I)-catalyzed cycloisomerization of N-propargyl aniline and construction of the azepinone core through the acid-catalyzed lactamization of an aminophenyl cyanoquinoline precursor, which was prepared using a simple umpolung approach that involves regioselective bromination followed by the Rosenmund-von Braun reaction.
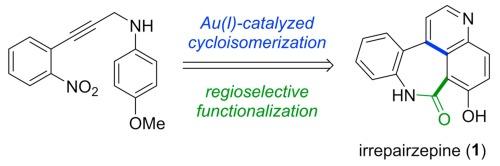
通过金(I)催化的环异构化和区域选择性官能化全合成天然苯并氮杂卓喹啉酮生物碱伊瑞西平
从一种内生真菌中分离出的一种独特的苯并氮杂卓喹啉酮生物碱--伊瑞西平(IRREPZEPINE)(1),在缺乏 PTEN 的胶质母细胞瘤细胞中表现出合成致死性。在此,我们通过一系列高产反应首次合成了伊瑞西平。关键步骤包括通过 Au(I)-catalyzed cycloisomerization of N-propargyl aniline(N-丙炔基苯胺的 Au(I)-catalyzed cycloisomerization)合成喹啉,以及通过酸催化氨基苯基氰基喹啉前体的内酰胺化来构建氮杂环庚酮核心。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Tetrahedron Letters
化学-有机化学
CiteScore
3.50
自引率
5.60%
发文量
521
审稿时长
28 days
期刊介绍:
Tetrahedron Letters provides maximum dissemination of outstanding developments in organic chemistry. The journal is published weekly and covers developments in techniques, structures, methods and conclusions in experimental and theoretical organic chemistry. Rapid publication of timely and significant research results enables researchers from all over the world to transmit quickly their new contributions to large, international audiences.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


