Patterned glycopeptide-based supramolecular hydrogel promotes the alignment and contractility of iPSC-derived cardiomyocytes
IF 5.5
2区 医学
Q2 MATERIALS SCIENCE, BIOMATERIALS
Materials Science & Engineering C-Materials for Biological Applications
Pub Date : 2024-10-29
DOI:10.1016/j.bioadv.2024.214091
引用次数: 0
Abstract
The functional restoration of a damaged cardiac tissue relies on a synchronized contractile capacity of exogenous and/or endogenous cardiomyocytes, which is challenging to achieve. Here, we explored the potential of the short glycopeptide diphenylalanine glucosamine-6-sulfate (FFGlcN6S) conjugated with an aromatic moiety, namely fluorenylmethoxycarbonyl (Fmoc), to enhance cardiac tissue regeneration. At physiological conditions, Fmoc-FFGlcN6S assembles into nanofibrous hydrated meshes, i.e., matrix mimicking hydrogels. These hydrogels can be further micropatterned allowing co-existence of hierarchical structures at different lenght. The patterned hydrogels support the culture of induced pluripotent stem cell-derived cardiomyocytes (iPSC-CMs) and promote their alignment. The cultured iPSC-CMs exhibit anisotropic synchronized contractions, indicating maturation and electrical interconnectivity. Moreover, the cultures express specific cardiac markers including, connexin-43 and sarcomeric-α-actinin, confirming enhanced cell-cell crosstalk, spontaneous contractility, and efficient transmission of electrical signals. Our results showcase the potential of short amphiphilic glycopeptides to mimic physical and biochemical cues that are essential for cardiomyocytes functionality and thus, these conjugates can be used in cardiac tissue engineering and regeneration.
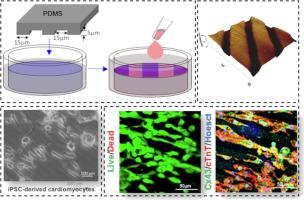
基于糖肽的图案化超分子水凝胶可促进 iPSC 衍生心肌细胞的排列和收缩能力
受损心脏组织的功能恢复有赖于外源性和/或内源性心肌细胞的同步收缩能力,而要实现这一点却很困难。在这里,我们探索了短糖肽二苯丙氨酸葡糖胺-6-硫酸盐(FFGlcN6S)与芳香族分子(即芴甲氧羰基(Fmoc))共轭的潜力,以促进心脏组织再生。在生理条件下,Fmoc-FFGlcN6S 可组装成纳米纤维状水合网,即基质模拟水凝胶。这些水凝胶可以进一步微图案化,从而实现不同长度的分层结构共存。图案化水凝胶支持诱导多能干细胞衍生心肌细胞(iPSC-CMs)的培养,并促进其排列。培养出的 iPSC-CMs 表现出各向异性的同步收缩,表明其已经成熟并具有电互联性。此外,培养物还能表达特定的心脏标记物,包括连接蛋白-43 和肌纤维-α-肌动蛋白,这证明细胞间的串联、自发收缩能力和电信号的有效传输都得到了增强。我们的研究结果展示了短两亲糖肽模拟对心肌细胞功能至关重要的物理和生化线索的潜力,因此这些共轭物可用于心脏组织工程和再生。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
17.80
自引率
0.00%
发文量
501
审稿时长
27 days
期刊介绍:
Biomaterials Advances, previously known as Materials Science and Engineering: C-Materials for Biological Applications (P-ISSN: 0928-4931, E-ISSN: 1873-0191). Includes topics at the interface of the biomedical sciences and materials engineering. These topics include:
• Bioinspired and biomimetic materials for medical applications
• Materials of biological origin for medical applications
• Materials for "active" medical applications
• Self-assembling and self-healing materials for medical applications
• "Smart" (i.e., stimulus-response) materials for medical applications
• Ceramic, metallic, polymeric, and composite materials for medical applications
• Materials for in vivo sensing
• Materials for in vivo imaging
• Materials for delivery of pharmacologic agents and vaccines
• Novel approaches for characterizing and modeling materials for medical applications
Manuscripts on biological topics without a materials science component, or manuscripts on materials science without biological applications, will not be considered for publication in Materials Science and Engineering C. New submissions are first assessed for language, scope and originality (plagiarism check) and can be desk rejected before review if they need English language improvements, are out of scope or present excessive duplication with published sources.
Biomaterials Advances sits within Elsevier''s biomaterials science portfolio alongside Biomaterials, Materials Today Bio and Biomaterials and Biosystems. As part of the broader Materials Today family, Biomaterials Advances offers authors rigorous peer review, rapid decisions, and high visibility. We look forward to receiving your submissions!

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


