3D printer fabrication of boron doped Cu-MWCNT/PLA electrodes: Investigation of its efficiency in electrocatalytic hydrogen production
IF 4.3
3区 材料科学
Q2 MATERIALS SCIENCE, COATINGS & FILMS
引用次数: 0
Abstract
In this study, three-dimensional (3D) electrodes based on MWCNT-Cu/PLA with high electrocatalytic efficiency, large surface area and different amounts of nano‑boron doped MWCNT-Cu/PLA were fabricated using Fused Granular Fabrication (FGF) method with a 3D printer. When FE-SEM images are examined, MWCNT fibers and copper particles are seen more clearly on the surface due to the formation of more dense conductive networks between the conductive carbon fibers trapped in the PLA structure due to the increasing amount of boron. When XRD results are examined, it is observed that the characteristic peaks belonging to the face-centered cubic structure of Cu are dominant and the crystal size increases as MWCNT addition and boron doping increase. When Raman spectra were analyzed to investigate the internal structural changes of MWCNTs, three characteristic bands corresponding to the D band (defect), G band (graphite band) and G' band (D overtone) of MWCNTs were determined and it was observed that the ID/IG ratio decreased with increasing boron doping compared to MWCNT-Cu/PLA electrode. The B1s spectrum of the 3D 0.8B@MWCNT-Cu/PLA electrode confirmed the presence of boron with the peak corresponding to B@C bonds at 191.2 eV. The obtained 3D electrocatalysts were used as cathodes in an electrolysis cell in aqueous solution containing 1 M KOH and their catalytic efficiency in hydrogen evolution reaction (HER) was tested. In order to increase the activity of the 3D electrodes, unlike the literature, they were activated by electrochemical activation process without using organic solvent and HER measurements of the activated electrodes were performed. Before activation, the charge transfer resistance (Rct) value of the Cu/PLA electrode, which was 6219.00 Ω cm−2, decreased to 681.90 Ω cm−2 after 5 % MWCNT doping. It is seen that with boron doping, a greater decrease in Rct value is realized, reaching values of 66.50–74.56 Ω cm−2. The cathodic Tafel slopes show that the Volmer reaction is the rate-determining step for HER and the rate-determining step is the electrochemical adsorption of hydrogen on the metal surface. In addition, energy efficiency tests of the 3D electrodes in HER were also performed and electrochemically active surface areas were determined. Thus, this study has taken an important step toward using 3D electrocatalysts, which are produced with more cost and more flexible designs, unlike previous production techniques, for hydrogen energy production.
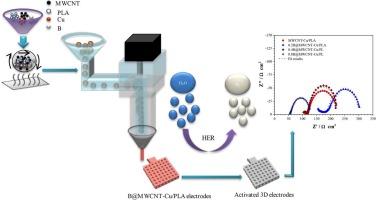
用三维打印机制造掺硼的 Cu-MWCNT/PLA 电极:电催化制氢效率研究
本研究利用三维打印机,采用熔融颗粒制造(FGF)方法,制造了基于 MWCNT-Cu/PLA 的三维(3D)电极,该电极具有高电催化效率、大表面积和不同掺杂量的纳米硼MWCNT-Cu/PLA。在观察 FE-SEM 图像时,由于硼含量的增加,在聚乳酸结构中的导电碳纤维之间形成了更致密的导电网络,因此在表面可以更清晰地看到 MWCNT 纤维和铜颗粒。在研究 XRD 结果时,可以观察到属于铜的面心立方结构的特征峰占主导地位,并且晶体尺寸随着 MWCNT 添加量和硼掺杂量的增加而增大。在分析拉曼光谱以研究 MWCNT 的内部结构变化时,确定了与 MWCNT-Cu/PLA电极的 D 带(缺陷)、G 带(石墨带)和 G'带(D 泛音)相对应的三个特征带,并观察到与 MWCNT-Cu/PLA 电极相比,ID/IG 比随着硼掺杂量的增加而降低。三维 0.8B@MWCNT-Cu/PLA 电极的 B1s 光谱证实了硼的存在,对应于 B@C 键的峰值为 191.2 eV。将获得的三维电催化剂用作电解池的阴极,在含有 1 M KOH 的水溶液中测试其在氢进化反应(HER)中的催化效率。为了提高三维电极的活性,与文献不同的是,这些电极是在不使用有机溶剂的情况下通过电化学活化过程活化的。活化前,Cu/PLA 电极的电荷转移电阻(Rct)值为 6219.00 Ω cm-2,掺杂 5 % MWCNT 后降至 681.90 Ω cm-2。可以看出,随着硼的掺入,Rct 值的下降幅度更大,达到了 66.50-74.56 Ω cm-2。阴极 Tafel 斜率表明,Volmer 反应是 HER 的速率决定步骤,而速率决定步骤是氢在金属表面的电化学吸附。此外,还对三维电极在 HER 中的能效进行了测试,并确定了电化学活性表面积。因此,与以往的生产技术不同,三维电催化剂的生产成本更低、设计更灵活,这项研究为利用三维电催化剂生产氢能迈出了重要一步。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Diamond and Related Materials
工程技术-材料科学:综合
CiteScore
6.00
自引率
14.60%
发文量
702
审稿时长
2.1 months
期刊介绍:
DRM is a leading international journal that publishes new fundamental and applied research on all forms of diamond, the integration of diamond with other advanced materials and development of technologies exploiting diamond. The synthesis, characterization and processing of single crystal diamond, polycrystalline films, nanodiamond powders and heterostructures with other advanced materials are encouraged topics for technical and review articles. In addition to diamond, the journal publishes manuscripts on the synthesis, characterization and application of other related materials including diamond-like carbons, carbon nanotubes, graphene, and boron and carbon nitrides. Articles are sought on the chemical functionalization of diamond and related materials as well as their use in electrochemistry, energy storage and conversion, chemical and biological sensing, imaging, thermal management, photonic and quantum applications, electron emission and electronic devices.
The International Conference on Diamond and Carbon Materials has evolved into the largest and most well attended forum in the field of diamond, providing a forum to showcase the latest results in the science and technology of diamond and other carbon materials such as carbon nanotubes, graphene, and diamond-like carbon. Run annually in association with Diamond and Related Materials the conference provides junior and established researchers the opportunity to exchange the latest results ranging from fundamental physical and chemical concepts to applied research focusing on the next generation carbon-based devices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


