Effects of Korean Red Ginseng combination therapy on HIV-infected patients treated with integrase strand transfer inhibitors
IF 6.8
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Background
Korean Red Ginseng (KRG) combined with antiretroviral therapy (ART) has shown benefits in the treatment of HIV-1-infected patients. Current guidelines recommend regimens containing integrase strand transfer inhibitors (INSTIs) as first-line treatment for these patients. The present study assessed the duration of effectiveness of ginseng combination therapy (GCT) in patients receiving INSTIs.
Methods
This study included 58 HIV-1-infected patients previously untreated with monotherapy or two-drug combination therapy. Patients in the GCT (n = 26) group received ART plus KRG for 164 ± 64 months, whereas patients in the control (n = 32) group received ART alone for 128 ± 49 months. Subsequently, patients in these two groups received INSTI for 81 ± 36 months and 68 ± 26 months, respectively.
Results
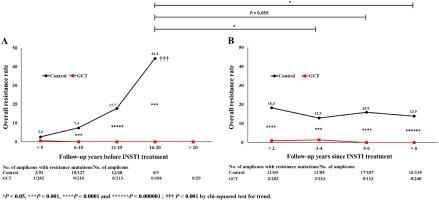
Before INSTI treatment, only one drug resistance mutation (DRM) was observed in the GCT group, compared with an overall resistance rate of 44.4 % in the control group (P < 0.001). The overall resistance rate was higher in the control than in the GCT group (9.5 % vs. 0.12 %, P < 0.001). During INSTI treatment, the resistance rate in the GCT group remained 0 % for over 5 years, but gradually decreased in the control group from 18.3 % to 13.9 % over 6 years, indicating that the between-group difference in resistance rate gradually decreased during INSTI treatment.
Conclusion
The beneficial effects of KRG were well maintained for more than 20 years, including the INSTI treatment period.
高丽红参联合疗法对接受整合酶链转移抑制剂治疗的艾滋病毒感染者的影响
背景韩国红参(KRG)与抗逆转录病毒疗法(ART)联合使用,在治疗 HIV-1 感染者方面效果显著。现行指南建议将含有整合酶链转移抑制剂(INSTIs)的治疗方案作为这些患者的一线治疗。本研究评估了人参联合疗法(GCT)对接受INSTIs治疗的患者的疗效持续时间。GCT组(n = 26)患者接受抗逆转录病毒疗法加KRG治疗164 ± 64个月,而对照组(n = 32)患者仅接受抗逆转录病毒疗法128 ± 49个月。结果在 INSTI 治疗前,GCT 组仅观察到一个耐药突变(DRM),而对照组的总体耐药率为 44.4%(P <0.001)。对照组的总体耐药率高于 GCT 组(9.5% 对 0.12%,P <0.001)。在 INSTI 治疗期间,GCT 组的耐药率在 5 年多的时间里一直保持为 0%,而对照组则在 6 年多的时间里从 18.3% 逐渐降至 13.9%,这表明耐药率的组间差异在 INSTI 治疗期间逐渐缩小。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Ginseng Research
CHEMISTRY, MEDICINAL-INTEGRATIVE & COMPLEMENTARY MEDICINE
CiteScore
11.40
自引率
9.50%
发文量
111
审稿时长
6-12 weeks
期刊介绍:
Journal of Ginseng Research (JGR) is an official, open access journal of the Korean Society of Ginseng and is the only international journal publishing scholarly reports on ginseng research in the world. The journal is a bimonthly peer-reviewed publication featuring high-quality studies related to basic, pre-clinical, and clinical researches on ginseng to reflect recent progresses in ginseng research.
JGR publishes papers, either experimental or theoretical, that advance our understanding of ginseng science, including plant sciences, biology, chemistry, pharmacology, toxicology, pharmacokinetics, veterinary medicine, biochemistry, manufacture, and clinical study of ginseng since 1976. It also includes the new paradigm of integrative research, covering alternative medicinal approaches. Article types considered for publication include review articles, original research articles, and brief reports.
JGR helps researchers to understand mechanisms for traditional efficacy of ginseng and to put their clinical evidence together. It provides balanced information on basic science and clinical applications to researchers, manufacturers, practitioners, teachers, scholars, and medical doctors.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


