Mitochondria/lysosome dual-organelle labelling esterase probe for monitoring cell viability and evaluating lung cancer drug efficiency
IF 4.3
2区 化学
Q1 SPECTROSCOPY
Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy
Pub Date : 2024-11-02
DOI:10.1016/j.saa.2024.125379
引用次数: 0
Abstract
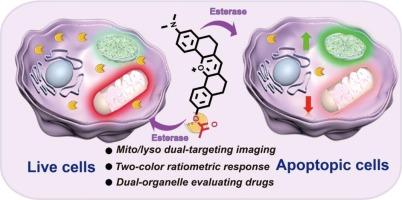
Monitoring of cell viability plays a key role in cancer therapy and evaluation of drug efficiency. Mitochondria and lysosomes are involved in regulating cell viability in many biological processes such as apoptosis, necrosis, autophagy, and cell proliferation. Thus, there is an emerging interest in the real-time evaluation of cell viability in both mitochondria and lysosomes. Herein, for the first time, we rationally designed and developed a mitochondria/lysosome dual-organelle labelling esterase-responsive ratiometric fluorescent probe, named TMLE-2, for dual-channel monitoring of cell viability and evaluation of lung cancer drug efficiency. TMLE-2 showed dramatic ratio fluorescence changes (about 51-fold) upon reacting with esterase. Furthermore, TMLE-2 enabled visualization of mitochondria and lysosomes with red and green emission, respectively; moreover, H2O2-induced cell damage, sorafenib-induced ferroptosis and ascorbic-acid-mediated cell protective effects were successfully assessed by dual-organelle ratiometric fluorescent imaging and flow cytometry data. More importantly, TMLE-2 was successfully used for the first time to evaluate the efficiency of lung cancer drugs at the cellular and tissue levels based on dual-organelle esterase activity assay. In summary, the newly designed TMLE-2 is expected to have enormous potential for facilitating advancements in biomedical fields related to cell viability.
用于监测细胞活力和评估肺癌药物疗效的线粒体/溶酶体双细胞器标记酯酶探针
监测细胞活力在癌症治疗和药物效率评估中起着关键作用。线粒体和溶酶体在细胞凋亡、坏死、自噬和细胞增殖等许多生物过程中参与调节细胞活力。因此,对线粒体和溶酶体的细胞活力进行实时评估的兴趣日渐浓厚。在此,我们首次合理设计并开发了一种线粒体/溶酶体双细胞器标记酯酶响应比率荧光探针,命名为TMLE-2,用于双通道监测细胞活力和评估肺癌药物效率。TMLE-2 与酯酶反应后,其比率荧光发生了显著变化(约 51 倍)。此外,TMLE-2 还能以红色和绿色发射分别显示线粒体和溶酶体;而且,H2O2 诱导的细胞损伤、索拉非尼诱导的铁变态反应以及抗坏血酸介导的细胞保护效应都能通过双细胞器比率荧光成像和流式细胞仪数据得到成功评估。更重要的是,基于双细胞器酯酶活性检测,TMLE-2 首次成功用于评估肺癌药物在细胞和组织水平的疗效。总之,新设计的 TMLE-2 在促进与细胞活力相关的生物医学领域的发展方面具有巨大潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
8.40
自引率
11.40%
发文量
1364
审稿时长
40 days
期刊介绍:
Spectrochimica Acta, Part A: Molecular and Biomolecular Spectroscopy (SAA) is an interdisciplinary journal which spans from basic to applied aspects of optical spectroscopy in chemistry, medicine, biology, and materials science.
The journal publishes original scientific papers that feature high-quality spectroscopic data and analysis. From the broad range of optical spectroscopies, the emphasis is on electronic, vibrational or rotational spectra of molecules, rather than on spectroscopy based on magnetic moments.
Criteria for publication in SAA are novelty, uniqueness, and outstanding quality. Routine applications of spectroscopic techniques and computational methods are not appropriate.
Topics of particular interest of Spectrochimica Acta Part A include, but are not limited to:
Spectroscopy and dynamics of bioanalytical, biomedical, environmental, and atmospheric sciences,
Novel experimental techniques or instrumentation for molecular spectroscopy,
Novel theoretical and computational methods,
Novel applications in photochemistry and photobiology,
Novel interpretational approaches as well as advances in data analysis based on electronic or vibrational spectroscopy.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


