Lacticaseibacillus rhamnosus CCFM1060 Modulates gut microbiota and intestinal barrier Function: Alcoholic liver disease Mitigation through Nrf2/HO-1 and NF-κB Pathways
IF 4
2区 农林科学
Q2 FOOD SCIENCE & TECHNOLOGY
引用次数: 0
Abstract
Long-term and/or excessive ethanol intake can lead to alcoholic liver disease (ALD). Gut dysbiosis contributes to a critical role in the pathogenesis of ALD. Here, we explored the impact of viable and dead Lacticaseibacillus rhamnosus CCFM1060 on the gut microbiota of alcohol-treated mice. The findings indicated that CCFM1060 V and D (viable and dead) administration improved the gut microbiota composition. Specifically, CCFM1060 D restored the abundance of butyric acid-producing bacteria, Alistipes, Lachnospiraceae, and Ruminococcaceae in ethanol-induced mice; CCFM1060 V improved the abundance of g_Dubsiela, g_Bifdobacterium, g_Ruminococcaceae_UCG_014_2, the SCFA-producing bacteria which were decreased in alcoholic hepatitis. Furthermore, the interventions improved the microstructure of the ileum, including the villi and crypts. They reinforced the intestinal barrier by increasing the RNA and protein expression of claudin-1, occludin, and ZO-1 in the colon. Also, they have been shown to enhance the liver’s antioxidant function by suppressing the synthesis of MDA and boosting the concentrations of GSH and SOD through the Nrf2/HO-1 pathway. In addition, interventions can suppress mice’s levels of TNF-α and IL-6, IL-1β in serum, presumably via the TRL4/MyD88/NF-κB pathway. Together, these results suggest that CCFM1060 can modulate the gut microbiota structure, reinforce the intestinal barrier, improve intestinal homeostasis, enhance liver antioxidant capacity, and mitigate inflammation response, eventually protecting the liver from ethanol-induced injury.
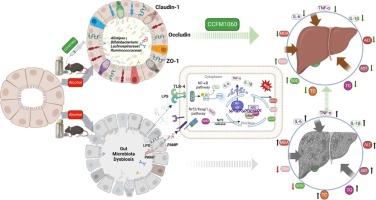
鼠李糖乳杆菌 CCFM1060 可调节肠道微生物群和肠屏障功能:通过 Nrf2/HO-1 和 NF-κB 途径缓解酒精性肝病
长期和/或过量摄入乙醇可导致酒精性肝病(ALD)。肠道菌群失调在酒精性肝病的发病机制中起着至关重要的作用。在此,我们探讨了有活力和死亡的鼠李糖乳杆菌 CCFM1060 对酒精处理小鼠肠道微生物群的影响。研究结果表明,施用CCFM1060 V和D(存活和死亡)可改善肠道微生物群的组成。具体而言,CCFM1060 D恢复了乙醇诱导小鼠体内丁酸产生菌、Alistipes、Lachnospiraceae和Ruminococcaceae的数量;CCFM1060 V改善了酒精性肝炎中减少的SCFA产生菌g_Dubsiela、g_Bifdobacterium、g_Ruminococcaceae_UCG_014_2的数量。此外,干预措施还改善了回肠的微观结构,包括绒毛和隐窝。它们通过增加结肠中 claudin-1、occludin 和 ZO-1 的 RNA 和蛋白质表达来加强肠道屏障。此外,它们还能通过 Nrf2/HO-1 途径抑制 MDA 的合成,提高 GSH 和 SOD 的浓度,从而增强肝脏的抗氧化功能。此外,干预措施还能抑制小鼠血清中TNF-α和IL-6、IL-1β的水平,这可能是通过TRL4/MyD88/NF-κB途径实现的。这些结果表明,CCFM1060可调节肠道微生物群结构,强化肠道屏障,改善肠道平衡,提高肝脏抗氧化能力,减轻炎症反应,最终保护肝脏免受乙醇诱导的损伤。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Functional Foods
FOOD SCIENCE & TECHNOLOGY-
CiteScore
9.60
自引率
1.80%
发文量
428
审稿时长
76 days
期刊介绍:
Journal of Functional Foods continues with the same aims and scope, editorial team, submission system and rigorous peer review. We give authors the possibility to publish their top-quality papers in a well-established leading journal in the food and nutrition fields. The Journal will keep its rigorous criteria to screen high impact research addressing relevant scientific topics and performed by sound methodologies.
The Journal of Functional Foods aims to bring together the results of fundamental and applied research into healthy foods and biologically active food ingredients.
The Journal is centered in the specific area at the boundaries among food technology, nutrition and health welcoming papers having a good interdisciplinary approach. The Journal will cover the fields of plant bioactives; dietary fibre, probiotics; functional lipids; bioactive peptides; vitamins, minerals and botanicals and other dietary supplements. Nutritional and technological aspects related to the development of functional foods and beverages are of core interest to the journal. Experimental works dealing with food digestion, bioavailability of food bioactives and on the mechanisms by which foods and their components are able to modulate physiological parameters connected with disease prevention are of particular interest as well as those dealing with personalized nutrition and nutritional needs in pathological subjects.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


