Dynamic ammonium retention for nutrient separation from manure digestate
IF 7.1
2区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
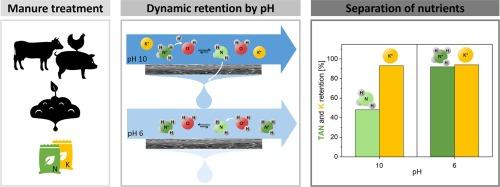
Extensive nitrogen emissions with negative impact on nature and the environment urge effective valorization of manure and fractionation of nutrients to enable precision fertilization. Typically, manure is fed to a digester to produce biogas. The remaining digestate is then mechanically separated into a solid phosphorous-rich fraction and a liquid fraction containing both NH4+ and K. These ions are difficult to separate due to their very identical size and charge. We show that with smart tuning of the pH to control the NH3/NH4+ equilibrium, membranes can produce dedicated N and K-rich streams. The increased pH switches the equilibrium towards the neutrally charged solute NH3 that permeates more easily through the membrane than charged NH4+ and K+ ions. Experiments with both artificial NH4Cl/KCl mixtures as well as real liquid digestate and four different membrane types, ranging from open nanofiltration (NF) to sea water reverse osmosis (RO) membranes were performed. At neutral pH, no N/K selectivity was observed, not for single components nor for mixtures. When the pH was increased towards alkaline environment, distinct selectivity for N/K was obtained both with model solutions and real liquid digestate. At a suitable pH of 10, with >80 % of the total ammonia present as NH3, the RO BW membrane showed a large N/K selectivity of 35 in the crossflow system. Additional RO steps at low pH allows subsequent concentration of the formed NH4+ and K+ fractions. The presented dynamic pH approach proofs that in a two-step RO system both N, and K-enriched fertilizers can be produced from real liquid digestate.
从粪便沼渣中分离养分的动态氨截留法
大量的氮排放会对自然和环境造成负面影响,因此需要对粪便进行有效的价值评估和养分分馏,以实现精准施肥。通常,粪便被送入沼气池产生沼气。剩余的沼渣通过机械分离成富含磷的固体部分和同时含有 NH4+ 和 K 的液体部分。我们的研究表明,通过巧妙地调节 pH 值来控制 NH3/NH4+ 的平衡,膜可以产生专用的富含 N 和 K 的液流。pH 值的升高会使平衡转向带中性电荷的溶质 NH3,它比带电荷的 NH4+ 和 K+ 离子更容易透过膜。实验中使用了人工 NH4Cl/KCl 混合物以及真正的液态沼渣和四种不同类型的膜,包括开放式纳滤(NF)膜和海水反渗透(RO)膜。在中性 pH 值下,无论是单一成分还是混合物,都没有观察到 N/K 选择性。当 pH 值升高到碱性环境时,模型溶液和实际液体消化物对 N/K 都有明显的选择性。在合适的 pH 值为 10 时,氨总量的 80% 以 NH3 形式存在,反渗透 BW 膜在横流系统中对 N/K 的选择性高达 35。在低 pH 值条件下进行额外的反渗透步骤,可以对形成的 NH4+ 和 K+ 部分进行后续浓缩。所介绍的动态 pH 值方法证明,在两步反渗透系统中,可以从真正的液态沼渣中生产出富含 N 和 K 的肥料。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Waste management
环境科学-工程:环境
CiteScore
15.60
自引率
6.20%
发文量
492
审稿时长
39 days
期刊介绍:
Waste Management is devoted to the presentation and discussion of information on solid wastes,it covers the entire lifecycle of solid. wastes.
Scope:
Addresses solid wastes in both industrialized and economically developing countries
Covers various types of solid wastes, including:
Municipal (e.g., residential, institutional, commercial, light industrial)
Agricultural
Special (e.g., C and D, healthcare, household hazardous wastes, sewage sludge)
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


