Spectroscopic Studies on Maillard Reactions of Peanut Ara h 2.01 With Respective Reducing Sugars and Their Impacts on Ara h 2.01 Allergenicity
Abstract
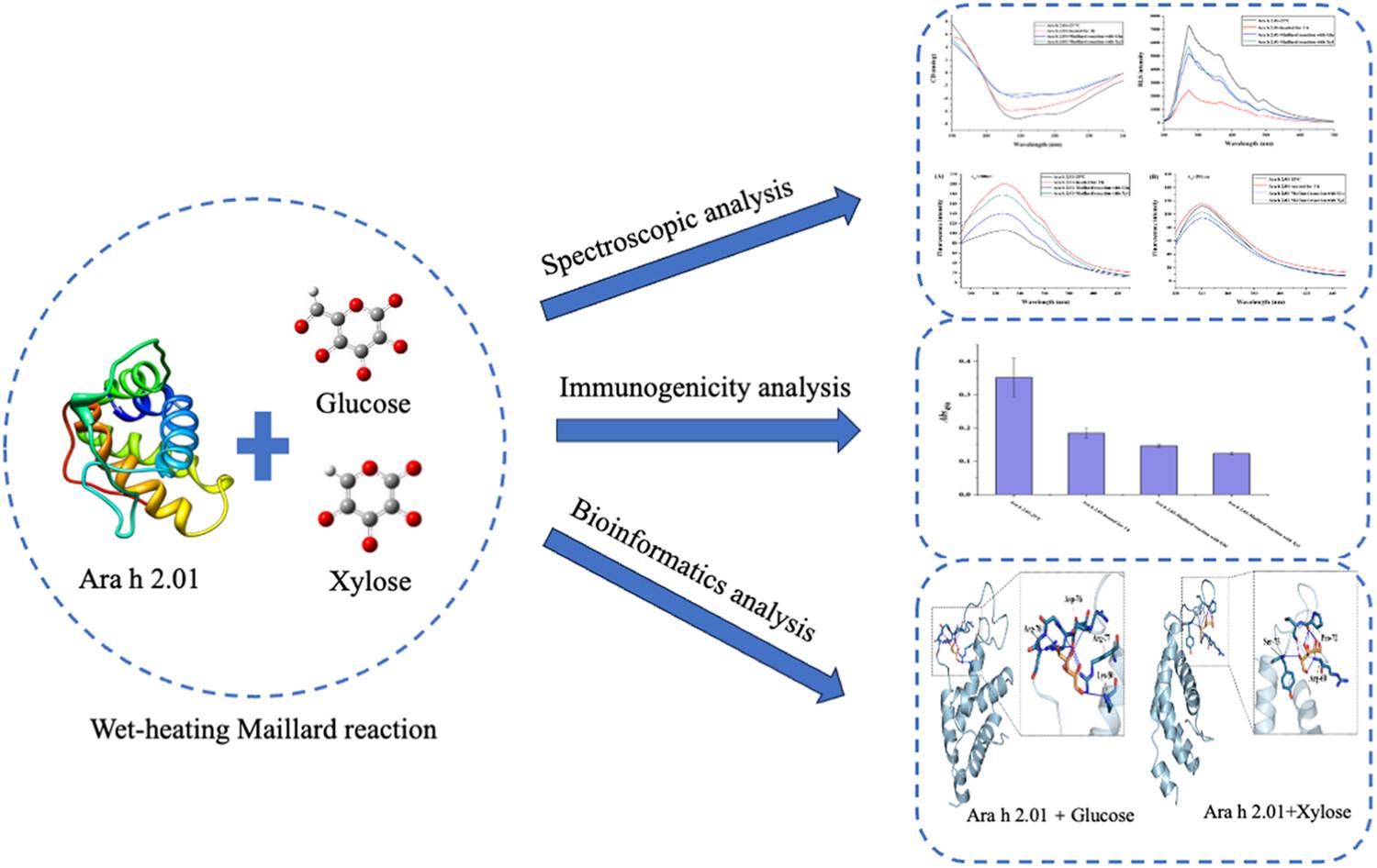
The effects of the Maillard reactions of peanut Ara h 2.01 with respective glucose and xylose on its conformation and allergenicity were investigated. Circular dichroism (CD) spectral studies showed that short-term heating alone with reducing sugars did not change protein secondary structures, but after undergoing Maillard reactions, its secondary structures changed with some α-helices being transformed into β-sheets and random coils. Fluorescence spectral studies indicated that as temperature increased, protein became loose and extended induced by the reducing sugars. Long heat treatment at higher temperature caused protein collapse with the extrusion of its internal water molecules, which was hindered partially by the reducing sugars that entered the protein in Maillard reaction. Resonance light scattering (RLS) spectra confirmed that more xylose molecules entered protein due to its stronger interaction with the protein after Maillard reactions. ELISA assays exhibited that heat treatment at 100°C led to some allergenic epitopes being embedded inside protein molecules due to protein collapse and that partial epitopes were covered by the reducing sugars after Maillard reaction, resulting in decreases in its allergenicity. Also, xylose reduced the allergenicity of protein more efficiently owing to the stronger interaction between them. All above experimental results were explained reasonably by bioinformatic analysis and molecular docking.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


