Engineering a xylose fermenting yeast for lignocellulosic ethanol production
IF 12.9
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
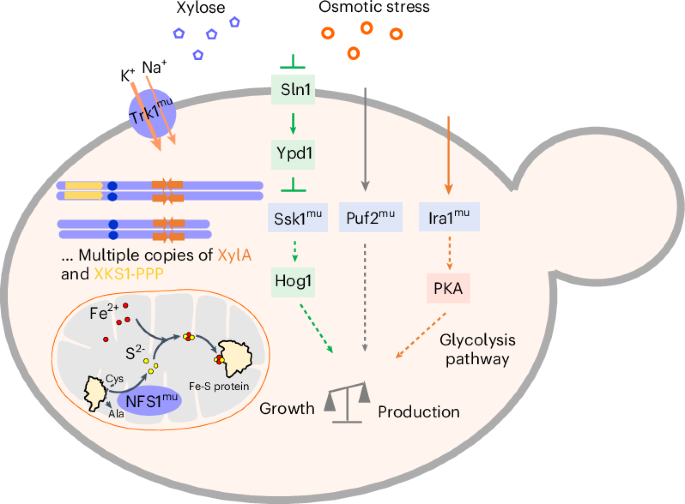
Lignocellulosic ethanol is produced by yeast fermentation of lignocellulosic hydrolysates generated by chemical pretreatment and enzymatic hydrolysis of plant cell walls. The conversion of xylose into ethanol in hydrolysates containing microbial inhibitors is a major bottleneck in biofuel production. We identified sodium salts as the primary yeast inhibitors, and evolved a Saccharomyces cerevisiae strain overexpressing xylose catabolism genes in xylose or glucose-mixed medium containing sodium salts. The fully evolved yeast strain can efficiently convert xylose in the hydrolysates to ethanol on an industrial scale. We elucidated that the amplification of xylA, XKS1 and pentose phosphate pathway-related genes TAL1, RPE1, TKL1, RKI1, along with mutations in NFS1, TRK1, SSK1, PUF2 and IRA1, are responsible and sufficient for the effective xylose utilization in corn stover hydrolysates containing high sodium salts. Our evolved or reverse-engineered yeast strains enable industrial-scale production of lignocellulosic ethanol and the genetic foundation we uncovered can also facilitate transfer of the phenotype to yeast cell factories producing chemicals beyond ethanol. The sodium salts in corn stover hydrolysates have been identified as important inhibitors for cellulosic ethanol production. Adaptive evolution generated a robust yeast that converted both glucose and xylose into biofuel. Subsequent reverse engineering created a genetically defined yeast with equivalent performance to the evolved strain.

改造木糖发酵酵母以生产木质纤维素乙醇
木质纤维素乙醇是通过化学预处理和酶水解植物细胞壁产生的木质纤维素水解物进行酵母发酵生产的。在含有微生物抑制剂的水解物中,木糖转化为乙醇是生物燃料生产的一个主要瓶颈。我们发现钠盐是主要的酵母抑制剂,并在含有钠盐的木糖或葡萄糖混合培养基中培育出过表达木糖分解基因的酿酒酵母菌株。完全进化后的酵母菌株可在工业规模上将水解物中的木糖高效转化为乙醇。我们阐明了 xylA、XKS1 和磷酸戊糖途径相关基因 TAL1、RPE1、TKL1、RKI1 的扩增,以及 NFS1、TRK1、SSK1、PUF2 和 IRA1 的突变,是在含高钠盐的玉米秸秆水解物中有效利用木糖的原因和充分条件。我们进化的或逆向工程的酵母菌株能够以工业规模生产木质纤维素乙醇,我们发现的遗传基础还有助于将表型转移到生产乙醇以外化学品的酵母细胞工厂。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature chemical biology
生物-生化与分子生物学
CiteScore
23.90
自引率
1.40%
发文量
238
审稿时长
12 months
期刊介绍:
Nature Chemical Biology stands as an esteemed international monthly journal, offering a prominent platform for the chemical biology community to showcase top-tier original research and commentary. Operating at the crossroads of chemistry, biology, and related disciplines, chemical biology utilizes scientific ideas and approaches to comprehend and manipulate biological systems with molecular precision.
The journal embraces contributions from the growing community of chemical biologists, encompassing insights from chemists applying principles and tools to biological inquiries and biologists striving to comprehend and control molecular-level biological processes. We prioritize studies unveiling significant conceptual or practical advancements in areas where chemistry and biology intersect, emphasizing basic research, especially those reporting novel chemical or biological tools and offering profound molecular-level insights into underlying biological mechanisms.
Nature Chemical Biology also welcomes manuscripts describing applied molecular studies at the chemistry-biology interface due to the broad utility of chemical biology approaches in manipulating or engineering biological systems. Irrespective of scientific focus, we actively seek submissions that creatively blend chemistry and biology, particularly those providing substantial conceptual or methodological breakthroughs with the potential to open innovative research avenues. The journal maintains a robust and impartial review process, emphasizing thorough chemical and biological characterization.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


