Discovery of the threshold potential that triggers photochemical water oxidation with Ru(II) photosensitizers and MOx catalysts
IF 11.5
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
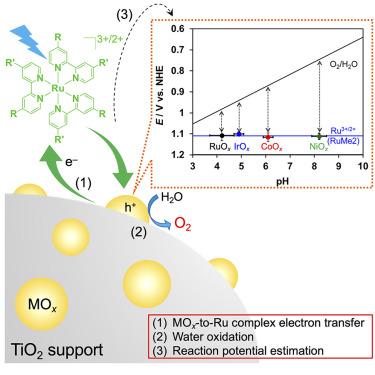
Photochemical water oxidation in the presence of a Ru(II) photosensitizer to form O2 is one of the most studied reactions in (photo)catalysis for both homogeneous and heterogeneous systems. In the present work, several Ru(II)-tris-diimine-type complexes with different ligands were used under a wide pH range (3.7–9.4) and over different transition-metal oxide (MOx) catalysts to reveal the factors that govern the O2 evolution activity. Most importantly, the results clarified that a certain “threshold” potential determines whether water oxidation can proceed and that this potential is related to the energy barrier for electron transfer from the MOx catalyst to the Ru(II) photosensitizer. The results of this work highlight that the potential of the electrons involved in the water oxidation on MOx catalysts can be estimated through the simple application of a photochemical reaction, which will be a useful measure for assessing the water oxidation activity of suspended nanoparticle catalysts.
利用 Ru(II) 光敏剂和 MOx 催化剂发现引发光化学水氧化的阈值电位
在 Ru(II) 光敏剂存在下进行光化学水氧化生成 O2 是研究最多的均相和异相系统(光)催化反应之一。在本研究中,研究人员在较宽的 pH 值范围(3.7-9.4)内,在不同的过渡金属氧化物(MOx)催化剂上使用了几种具有不同配体的 Ru(II)-tris-diimine 型配合物,以揭示影响 O2 演化活性的因素。最重要的是,研究结果明确了一个特定的 "阈值 "电位决定了水氧化能否进行,而这个电位与电子从 MOx 催化剂转移到 Ru(II) 光敏剂的能量障碍有关。这项工作的结果突出表明,可以通过简单的光化学反应来估算参与 MOx 催化剂上水氧化作用的电子的电位,这将是评估悬浮纳米粒子催化剂水氧化活性的有用指标。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
10.50
自引率
6.40%
发文量
0
期刊介绍:
Chem Catalysis is a monthly journal that publishes innovative research on fundamental and applied catalysis, providing a platform for researchers across chemistry, chemical engineering, and related fields. It serves as a premier resource for scientists and engineers in academia and industry, covering heterogeneous, homogeneous, and biocatalysis. Emphasizing transformative methods and technologies, the journal aims to advance understanding, introduce novel catalysts, and connect fundamental insights to real-world applications for societal benefit.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


