A novel Cu/Fe cathode prepared by a facile redox pathway for phenol degradation electrocatalytically via the electro-fenton assisted electro-chlorination process
IF 11.4
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
Electrochemical methods for treating phenolic wastewater have been widely studied, with most research focusing primarily on the anode, while the cathode has generally served as a counter electrode. This study aims to enhance the electrocatalytic process by developing a new Fe/Cu-based cathode using a simple redox method. We created a CuO![]() Cu@Fe-Fe2O3-x (0 < x < 1, combining Fe2O3 and FeO) electrode, referred to as CCFFO, to facilitate the electro-Fenton process without requiring additional H2O2 or Fe2+. In our electrolysis system with NaCl as the electrolyte for electro-chlorination process, phenol concentration was reduced from 100 mg/L to below 0.5 mg/L within 10 min. Optimal experimental conditions were determined by evaluating various parameters such as chloride electrolyte concentration, current density, electrode plate spacing, aeration, pH, and cathode types. Additionally, the role of chloride ions in phenol degradation was investigated through free radical quenching experiments. A 500-hour continuous flow experiment demonstrated the durability of the CCFFO cathode. GC/MS analysis identified intermediates formed during phenol degradation and the underlying catalytic mechanism was explored. The results indicate that the electro-chlorination process at the anode is the primary driver of phenol degradation, assisted by the electro-Fenton process on the CCFFO cathode. The CCFFO cathode effectively prevents the production of harmful by-products like perchlorate. The degradation efficiencies of chemical oxygen demand (COD) and total organic carbon (TOC) were 63.5 % and 80.25 %, respectively. Achieving a phenol degradation efficiency of 99.5 % within 10 min, the CCFFO cathode and electrolytic system show significant potential for wastewater treatment applications.
Cu@Fe-Fe2O3-x (0 < x < 1, combining Fe2O3 and FeO) electrode, referred to as CCFFO, to facilitate the electro-Fenton process without requiring additional H2O2 or Fe2+. In our electrolysis system with NaCl as the electrolyte for electro-chlorination process, phenol concentration was reduced from 100 mg/L to below 0.5 mg/L within 10 min. Optimal experimental conditions were determined by evaluating various parameters such as chloride electrolyte concentration, current density, electrode plate spacing, aeration, pH, and cathode types. Additionally, the role of chloride ions in phenol degradation was investigated through free radical quenching experiments. A 500-hour continuous flow experiment demonstrated the durability of the CCFFO cathode. GC/MS analysis identified intermediates formed during phenol degradation and the underlying catalytic mechanism was explored. The results indicate that the electro-chlorination process at the anode is the primary driver of phenol degradation, assisted by the electro-Fenton process on the CCFFO cathode. The CCFFO cathode effectively prevents the production of harmful by-products like perchlorate. The degradation efficiencies of chemical oxygen demand (COD) and total organic carbon (TOC) were 63.5 % and 80.25 %, respectively. Achieving a phenol degradation efficiency of 99.5 % within 10 min, the CCFFO cathode and electrolytic system show significant potential for wastewater treatment applications.
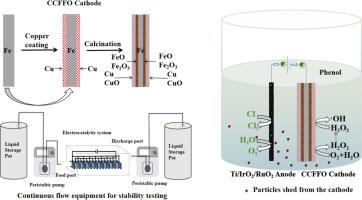
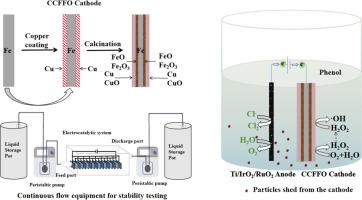
一种新型铜/铁阴极的制备方法,它采用简便的氧化还原途径,通过电-芬顿辅助电-氯化过程进行电催化降解苯酚
处理酚类废水的电化学方法已被广泛研究,大多数研究主要集中在阳极上,而阴极一般用作反电极。本研究旨在利用简单的氧化还原法开发一种新的铁/铜基阴极,从而增强电催化过程。我们创建了一种 CuO-Cu@Fe-Fe2O3-x (0 < x < 1,结合了 Fe2O3 和 FeO)电极,简称为 CCFFO,以促进电-芬顿过程,而无需额外的 H2O2 或 Fe2+。在以 NaCl 为电解质的电解系统中,苯酚浓度在 10 分钟内从 100 mg/L 降至 0.5 mg/L 以下。通过评估各种参数,如氯化物电解液浓度、电流密度、电极板间距、通气、pH 值和阴极类型,确定了最佳实验条件。此外,还通过自由基淬灭实验研究了氯离子在苯酚降解中的作用。500 小时的连续流动实验证明了 CCFFO 阴极的耐用性。气相色谱/质谱分析确定了苯酚降解过程中形成的中间产物,并探讨了潜在的催化机理。结果表明,阳极上的电氯化过程是苯酚降解的主要驱动力,CCFFO 阴极上的电-芬顿过程起到辅助作用。CCFFO 阴极可有效防止高氯酸盐等有害副产品的产生。化学需氧量(COD)和总有机碳(TOC)的降解效率分别为 63.5% 和 80.25%。CCFFO 阴极和电解系统在 10 分钟内的苯酚降解效率达到 99.5%,显示出在废水处理应用中的巨大潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Water Research
环境科学-工程:环境
CiteScore
20.80
自引率
9.40%
发文量
1307
审稿时长
38 days
期刊介绍:
Water Research, along with its open access companion journal Water Research X, serves as a platform for publishing original research papers covering various aspects of the science and technology related to the anthropogenic water cycle, water quality, and its management worldwide. The audience targeted by the journal comprises biologists, chemical engineers, chemists, civil engineers, environmental engineers, limnologists, and microbiologists. The scope of the journal include:
•Treatment processes for water and wastewaters (municipal, agricultural, industrial, and on-site treatment), including resource recovery and residuals management;
•Urban hydrology including sewer systems, stormwater management, and green infrastructure;
•Drinking water treatment and distribution;
•Potable and non-potable water reuse;
•Sanitation, public health, and risk assessment;
•Anaerobic digestion, solid and hazardous waste management, including source characterization and the effects and control of leachates and gaseous emissions;
•Contaminants (chemical, microbial, anthropogenic particles such as nanoparticles or microplastics) and related water quality sensing, monitoring, fate, and assessment;
•Anthropogenic impacts on inland, tidal, coastal and urban waters, focusing on surface and ground waters, and point and non-point sources of pollution;
•Environmental restoration, linked to surface water, groundwater and groundwater remediation;
•Analysis of the interfaces between sediments and water, and between water and atmosphere, focusing specifically on anthropogenic impacts;
•Mathematical modelling, systems analysis, machine learning, and beneficial use of big data related to the anthropogenic water cycle;
•Socio-economic, policy, and regulations studies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


