High-resolution cryo-EM analysis of a Streptococcus pyogenes M-protein/human plasminogen complex
IF 4.4
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
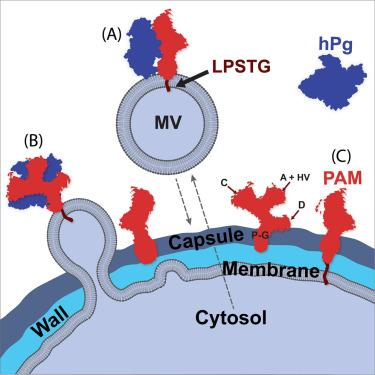
The importance of human plasminogen (hPg)/plasmin (hPm)/cell receptor complexes in invasiveness of cells has been amply established. The objective of this investigation was to determine a high-resolution structure of a major Group A Streptococcus (GAS) bacterial receptor (PAM) for hPg/hPm when bound on a cell surface to its major ligand, hPg. As a model cell surface with endogenous PAM, we employed engineered PAM-expressing lentivirus (LV) particles. We show that the ectodomain of a PAM-type M-Protein (M-Prt), in complex with hPg, is folded through distinct intra- and inter-domain interactions to a more compact form on the cell surface, thus establishing a new paradigm for membrane-bound M-Prt/ligand structures. These studies provide a framework for addressing the need for treatments of GAS disease by providing a molecular platform to solve structures of virulence-determining membrane proteins.
化脓性链球菌 M 蛋白/人纤溶酶原复合物的高分辨率冷冻电镜分析
人类纤溶酶原(hPg)/纤溶酶原(hPm)/细胞受体复合物对细胞侵袭性的重要性已得到充分证实。本研究的目的是确定 A 组链球菌(GAS)细菌主要受体(PAM)在细胞表面与其主要配体 hPg 结合时的高分辨率结构。我们的研究表明,PAM 型 M 蛋白(M-Prt)的外结构域与 hPg 复合物通过不同的域内和域间相互作用折叠成细胞表面更紧凑的形式,从而建立了膜结合 M-Prt/ 配体结构的新范例。这些研究提供了一个解决决定毒力的膜蛋白结构的分子平台,从而为满足治疗 GAS 疾病的需要提供了一个框架。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Structure
生物-生化与分子生物学
CiteScore
8.90
自引率
1.80%
发文量
155
审稿时长
3-8 weeks
期刊介绍:
Structure aims to publish papers of exceptional interest in the field of structural biology. The journal strives to be essential reading for structural biologists, as well as biologists and biochemists that are interested in macromolecular structure and function. Structure strongly encourages the submission of manuscripts that present structural and molecular insights into biological function and mechanism. Other reports that address fundamental questions in structural biology, such as structure-based examinations of protein evolution, folding, and/or design, will also be considered. We will consider the application of any method, experimental or computational, at high or low resolution, to conduct structural investigations, as long as the method is appropriate for the biological, functional, and mechanistic question(s) being addressed. Likewise, reports describing single-molecule analysis of biological mechanisms are welcome.
In general, the editors encourage submission of experimental structural studies that are enriched by an analysis of structure-activity relationships and will not consider studies that solely report structural information unless the structure or analysis is of exceptional and broad interest. Studies reporting only homology models, de novo models, or molecular dynamics simulations are also discouraged unless the models are informed by or validated by novel experimental data; rationalization of a large body of existing experimental evidence and making testable predictions based on a model or simulation is often not considered sufficient.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


