Non-invasive lipid panel of MASLD fibrosis transition underscores the role of lipoprotein sulfatides in hepatic immunomodulation
IF 27.7
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
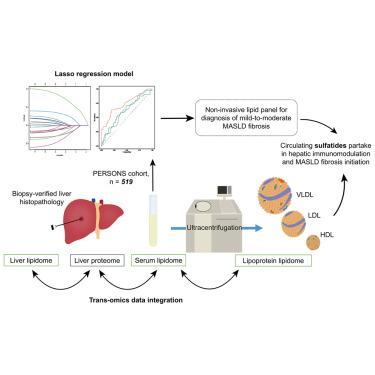
There exists a pressing need for a non-invasive panel that differentiates mild fibrosis from non-fibrosis in metabolic dysfunction-associated steatotic liver disease (MASLD). In this work, we applied quantitative lipidomics and sterolomics on sera from the PERSONS cohort with biopsy-based histological assessment of liver pathology. We trained a lasso regression model using quantitative omics data and clinical variables, deriving a combinatorial panel of lipids and clinical indices that differentiates mild fibrosis (>F1, n = 324) from non-fibrosis (F0, n = 195), with an area under receiver operating characteristic curve (AUROC) at 0.775 (95% confidence interval [CI]: 0.735–0.816). Circulating sulfatides (SLs) emerged as central lipids distinctly associated with fibrosis pathogenesis in MASLD. Lipidomics analysis of lipoprotein fractions revealed a redistribution of circulating SLs from high-density lipoproteins (HDLs) onto low-density lipoproteins (LDLs) in MASLD fibrosis. We further verified that patient LDLs with reduced SL content triggered a smaller activation of type II natural killer T lymphocytes, compared with control LDLs. Our results suggest that hepatic crosstalk with systemic immunity mediated by lipoprotein metabolism underlies fibrosis progression at early-stage MASLD.
MASLD纤维化转变的非侵入性脂质面板强调了脂蛋白硫化物在肝脏免疫调节中的作用
目前迫切需要一种非侵入性的检测方法来区分代谢功能障碍相关性脂肪性肝病(MASLD)中的轻度纤维化和非纤维化。在这项工作中,我们将定量脂质组学和固醇组学应用于 PERSONS 队列的血清,并通过活检对肝脏病理组织学进行评估。我们利用定量 omics 数据和临床变量训练了一个 lasso 回归模型,得出了一个能区分轻度纤维化(F1,n = 324)和非纤维化(F0,n = 195)的脂质和临床指数组合面板,接收者操作特征曲线下面积 (AUROC) 为 0.775(95% 置信区间 [CI]:0.735-0.816)。循环硫化物(SLs)是与 MASLD 纤维化发病机制明显相关的中心脂质。脂蛋白组分的脂质组学分析显示,在MASLD纤维化过程中,循环中的SLs从高密度脂蛋白(HDLs)重新分布到低密度脂蛋白(LDLs)上。我们进一步证实,与对照组低密度脂蛋白相比,SL 含量降低的患者低密度脂蛋白引发的 II 型自然杀伤 T 淋巴细胞活化较小。我们的研究结果表明,由脂蛋白代谢介导的肝脏与全身免疫之间的串扰是早期 MASLD 纤维化进展的基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell metabolism
生物-内分泌学与代谢
CiteScore
48.60
自引率
1.40%
发文量
173
审稿时长
2.5 months
期刊介绍:
Cell Metabolism is a top research journal established in 2005 that focuses on publishing original and impactful papers in the field of metabolic research.It covers a wide range of topics including diabetes, obesity, cardiovascular biology, aging and stress responses, circadian biology, and many others.
Cell Metabolism aims to contribute to the advancement of metabolic research by providing a platform for the publication and dissemination of high-quality research and thought-provoking articles.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


