Submicrometre spatiotemporal characterization of the Toxoplasma adhesion strategy for gliding motility
IF 19.4
1区 生物学
Q1 MICROBIOLOGY
引用次数: 0
Abstract
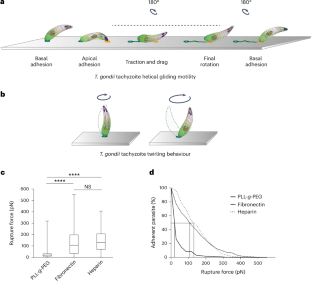
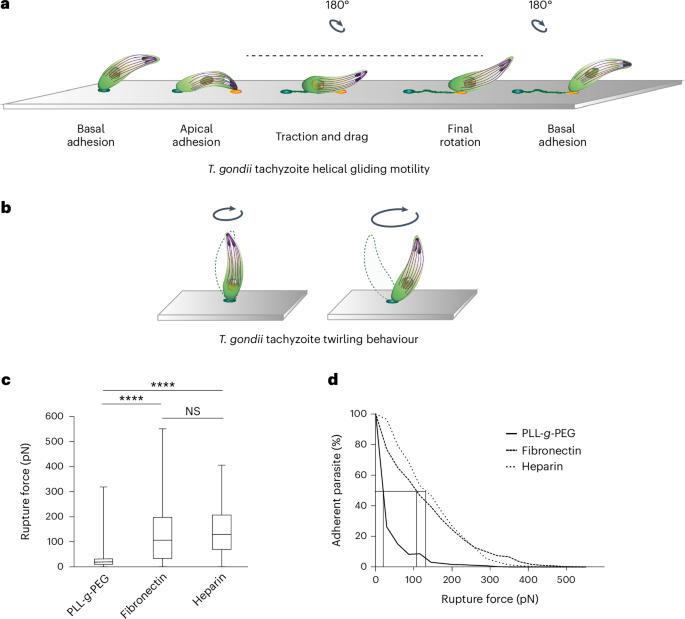
Toxoplasma gondii is a protozoan apicomplexan parasite that uses an adhesion-dependent mode of motility termed gliding to access host cells and disseminate into tissues. Previous studies on Apicomplexa motile morphotypes, including the T. gondii tachyzoite, have identified a cortical actin–myosin motor system that drives the rearward translocation of transmembrane adhesins, thus powering forward movement. However, this model is currently questioned. Here, combining micropatterning and tunable surface chemistry (to edit parasite surface ligands) with flow force and live or super-resolution imaging, we show that tachyzoites build only one apical anchoring contact with the substrate, over which it slides. Furthermore, we show that glycosaminoglycan–parasite interactions are sufficient to promote such force-productive contact and find that the apicobasal flow is set up independent of adhesin release and surface interactions. These findings should enable further characterization of the molecular functions at the T. gondii–substrate mechanosensitive interface and their comparison across apicomplexans. Live imaging, combined with micropatterning and tunable surface chemistry, reveals the adhesive strategy evolved by Toxoplasma gondii for helical gliding.
弓形虫滑行运动粘附策略的亚微米级时空特性分析
弓形虫(Toxoplasma gondii)是一种原生动物,它利用一种称为滑行的粘附依赖型运动模式进入宿主细胞并扩散到组织中。以前对包括淋病双球菌速殖体在内的棘球蚴运动形态的研究发现,皮质肌动蛋白-肌球蛋白运动系统可驱动跨膜粘附蛋白向后转移,从而为前向运动提供动力。然而,这一模型目前受到质疑。在这里,我们将微图案化和可调表面化学(编辑寄生虫表面配体)与流动力和实时或超分辨率成像相结合,证明鲎只与基质建立一个顶端锚定接触,然后在基质上滑动。此外,我们还发现,糖胺聚糖与寄生虫之间的相互作用足以促进这种产生力的接触,并发现尖基部流动的建立与粘附素释放和表面相互作用无关。这些发现将有助于进一步描述淋球菌-基质机械敏感界面的分子功能,并将其与其他类鼻疽进行比较。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Microbiology
Immunology and Microbiology-Microbiology
CiteScore
44.40
自引率
1.10%
发文量
226
期刊介绍:
Nature Microbiology aims to cover a comprehensive range of topics related to microorganisms. This includes:
Evolution: The journal is interested in exploring the evolutionary aspects of microorganisms. This may include research on their genetic diversity, adaptation, and speciation over time.
Physiology and cell biology: Nature Microbiology seeks to understand the functions and characteristics of microorganisms at the cellular and physiological levels. This may involve studying their metabolism, growth patterns, and cellular processes.
Interactions: The journal focuses on the interactions microorganisms have with each other, as well as their interactions with hosts or the environment. This encompasses investigations into microbial communities, symbiotic relationships, and microbial responses to different environments.
Societal significance: Nature Microbiology recognizes the societal impact of microorganisms and welcomes studies that explore their practical applications. This may include research on microbial diseases, biotechnology, or environmental remediation.
In summary, Nature Microbiology is interested in research related to the evolution, physiology and cell biology of microorganisms, their interactions, and their societal relevance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


