Tubulin acetyltransferases access and modify the microtubule luminal K40 residue through anchors in taxane-binding pockets
IF 12.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Acetylation at α-tubulin K40 is the sole post-translational modification preferred to occur inside the lumen of hollow cylindrical microtubules. However, how tubulin acetyltransferases access the luminal K40 in micrometer-long microtubules remains unknown. Here, we use cryo-electron microscopy and single-molecule reconstitution assays to reveal the enzymatic mechanism for tubulin acetyltransferases to modify K40 in the lumen. One tubulin acetyltransferase spans across the luminal lattice, with the catalytic core docking onto two α-tubulins and the enzyme’s C-terminal domain occupying the taxane-binding pockets of two β-tubulins. The luminal accessibility and enzyme processivity of tubulin acetyltransferases are inhibited by paclitaxel, a microtubule-stabilizing chemotherapeutic agent. Characterizations using recombinant tubulins mimicking preacetylated and postacetylated K40 show the crosstalk between microtubule acetylation states and the cofactor acetyl-CoA in enzyme turnover. Our findings provide crucial insights into the conserved multivalent interactions involving α- and β-tubulins to acetylate the confined microtubule lumen. Here, the authors reveal the mechanism by which anchors of a tubulin acetyltransferase in the β-tubulin taxane-binding pockets have critical roles in the enzyme luminal accessibility and processivity to modify α-tubulin K40 in the microtubule lumen.

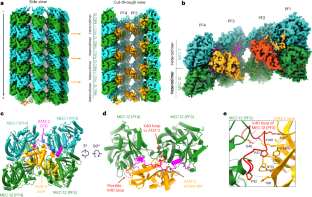
微管蛋白乙酰转移酶通过锚定在类黄酮结合袋中来获取和修改微管管腔内的 K40 残基
α-微管蛋白K40的乙酰化是中空圆柱形微管管腔内发生的唯一翻译后修饰。然而,微米长的微管中,管蛋白乙酰转移酶是如何进入管腔K40的仍是未知数。在这里,我们利用冷冻电镜和单分子重组实验揭示了管蛋白乙酰转移酶改变管腔中K40的酶学机制。一个小管蛋白乙酰转移酶横跨管腔晶格,其催化核心与两个α-小管蛋白对接,酶的C-末端结构域占据了两个β-小管蛋白的类固醇结合口袋。紫杉醇是一种微管稳定化疗药物,它能抑制管蛋白乙酰转移酶的管腔可及性和酶的加工性。使用模拟前乙酰化和后乙酰化 K40 的重组微管蛋白进行的表征显示,微管乙酰化状态和辅助因子乙酰-CoA 在酶转换过程中相互影响。我们的研究结果为了解α-和β-微管蛋白参与乙酰化封闭微管内腔的保守多价相互作用提供了重要见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Structural & Molecular Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-BIOPHYSICS
CiteScore
22.00
自引率
1.80%
发文量
160
审稿时长
3-8 weeks
期刊介绍:
Nature Structural & Molecular Biology is a comprehensive platform that combines structural and molecular research. Our journal focuses on exploring the functional and mechanistic aspects of biological processes, emphasizing how molecular components collaborate to achieve a particular function. While structural data can shed light on these insights, our publication does not require them as a prerequisite.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


