Cryo-EM structure and molecular mechanism of the jasmonic acid transporter ABCG16
IF 15.8
1区 生物学
Q1 PLANT SCIENCES
引用次数: 0
Abstract
Jasmonates (JAs) are a class of oxylipin phytohormones including jasmonic acid (JA) and derivatives that regulate plant growth, development and biotic and abiotic stress. A number of transporters have been identified to be responsible for the cellular and subcellular translocation of JAs. However, the mechanistic understanding of how these transporters specifically recognize and transport JAs is scarce. Here we determined the cryogenic electron microscopy structure of JA exporter AtABCG16 in inward-facing apo, JA-bound and occluded conformations, and outward-facing post translocation conformation. AtABCG16 structure forms a homodimer, and each monomer contains a nucleotide-binding domain, a transmembrane domain and an extracellular domain. Structural analyses together with biochemical and plant physiological experiments revealed the molecular mechanism by which AtABCG16 specifically recognizes and transports JA. Structural analyses also revealed that AtABCG16 features a unique bifurcated substrate translocation pathway, which is composed of two independent substrate entrances, two substrate-binding pockets and a shared apoplastic cavity. In addition, residue Phe608 from each monomer is disclosed to function as a gate along the translocation pathway controlling the accessing of substrate JA from the cytoplasm or apoplast. Based on the structural and biochemical analyses, a working model of AtABCG16-mediated JA transport is proposed, which diversifies the molecular mechanisms of ABC transporters. The authors report the cryo-EM structure of JA transporter ABCG16 in multiple conformations. It features a bifurcated translocation pathway, revealing the specific JA binding and transport mechanism that diversifies ABC transporters in higher plants.
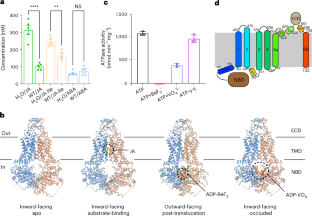

茉莉酸转运体 ABCG16 的冷冻电镜结构和分子机制
茉莉酸(JA)是一类氧化脂素植物激素,包括茉莉酸(JA)及其衍生物,可调节植物的生长、发育以及生物和非生物胁迫。目前已发现一些转运体负责 JA 的细胞和亚细胞转运。然而,人们对这些转运体如何特异性识别和转运 JA 的机理了解甚少。在这里,我们测定了JA转运体AtABCG16的低温电子显微镜结构,包括向内的apo构象、与JA结合的构象和闭锁构象,以及向外的转运后构象。AtABCG16 结构形成一个同源二聚体,每个单体包含一个核苷酸结合结构域、一个跨膜结构域和一个胞外结构域。结构分析以及生化和植物生理实验揭示了 AtABCG16 特异性识别和转运 JA 的分子机制。结构分析还揭示了 AtABCG16 独特的分叉底物转运途径,它由两个独立的底物入口、两个底物结合口袋和一个共享的凋亡腔组成。此外,每个单体的残基 Phe608 被披露为转运途径上的一个门,控制着底物 JA 从细胞质或细胞凋亡体的进入。基于结构和生化分析,提出了 AtABCG16 介导 JA 转运的工作模型,该模型使 ABC 转运体的分子机制多样化。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
Nature Plants
PLANT SCIENCES-
CiteScore
25.30
自引率
2.20%
发文量
196
期刊介绍:
Nature Plants is an online-only, monthly journal publishing the best research on plants — from their evolution, development, metabolism and environmental interactions to their societal significance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


