Removing low-concentration methane via thermo-catalytic oxidation on CuOx/zeolite
IF 6.3
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Methane (CH4) is the second most potent greenhouse gas that exists largely in low concentrations. This fact, coupled with its inert nature, brings both urgency and challenge for any mitigations (including thermo-catalytic oxidation). In this study, we address this challenge by synthesizing highly dispersed CuOx species (∼6 wt%) loaded on mordenite zeolite (MOR), and enhancing the catalytic performance for the thermal oxidation of low-concentration CH4. The optimized sample, Cu-MOR-11, demonstrates exceptional catalytic properties, including high activity with 100 % CH4 total oxidation to CO2 at 400 °C, low reaction temperature with a T10 at 230 °C and T90 at 350 °C, as well as excellent long-term stability and reusability over a 100-hour reaction period. These attributes make it a promising candidate for large scale CH4 oxidation applications. To elucidate the mechanisms behind the enhanced catalytic performance of Cu-MOR-11, we conclude, 1) the generation of more Brønsted acid sites which facilitated the absorption and dissociation of CH4; 2) the presence of Al3+ as acid sites in the MOR supports played a crucial role in achieving high CuOx species dispersion, acting as anchoring sites to effectively stabilize and disperse CuOx species, which provides more active sites; 3) variation in preparation environments (e.g., pH) led to different oxidation states of the catalysts, with alkaline conditions facilitating the deoxidation of CuOx species, resulting in more Cu+&Cu0 compared to CuO; 4) the presence of Brønsted acid sites which mitigated coking at low temperatures and prevented the loss of structural stability at high temperatures.
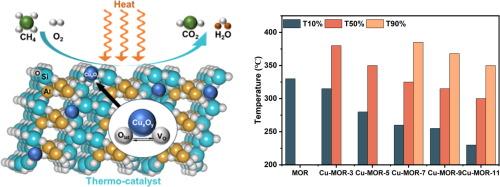
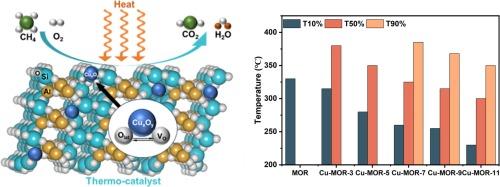
在氧化铜/沸石上通过热催化氧化去除低浓度甲烷
甲烷(CH4)是第二大强效温室气体,主要以低浓度存在。这一事实加上其惰性,给任何减排措施(包括热催化氧化)带来了紧迫性和挑战性。在本研究中,我们通过合成负载在莫来石沸石(MOR)上的高分散 CuOx 物种(∼6 wt%)来应对这一挑战,并提高低浓度 CH4 热氧化的催化性能。优化后的 Cu-MOR-11 样品具有优异的催化性能,包括高活性(400 ℃ 时可将 100% 的 CH4 全部氧化为 CO2)、低反应温度(T10 为 230 ℃,T90 为 350 ℃)以及出色的长期稳定性和 100 小时反应期的重复使用性。这些特性使其成为大规模甲烷氧化应用的理想候选物质。为了阐明 Cu-MOR-11 催化性能增强背后的机理,我们得出以下结论:1)更多布氏酸位点的产生促进了 CH4 的吸收和解离;2)MOR 支撑物中 Al3+ 作为酸位点的存在对实现 CuOx 物种的高度分散起到了至关重要的作用,它作为锚定位点有效地稳定和分散了 CuOx 物种,从而提供了更多的活性位点;3)制备环境(如 pH 值)的变化导致了不同的氧化态、3)制备环境(如 pH 值)的不同导致催化剂的氧化态不同,碱性条件有利于 CuOx 物种的脱氧,从而产生比 CuO 更多的 Cu+&Cu0;4)布氏酸位点的存在减轻了低温下的结焦,并防止了高温下结构稳定性的丧失。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Applied Surface Science
工程技术-材料科学:膜
CiteScore
12.50
自引率
7.50%
发文量
3393
审稿时长
67 days
期刊介绍:
Applied Surface Science covers topics contributing to a better understanding of surfaces, interfaces, nanostructures and their applications. The journal is concerned with scientific research on the atomic and molecular level of material properties determined with specific surface analytical techniques and/or computational methods, as well as the processing of such structures.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


