Determination of the apparent activation energy surface from isothermal data of char combustion and gasification
IF 3.1
2区 化学
Q2 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
In this paper, the apparent activation energy (AAE) surface was determined by using isothermal data of char combustion and gasification and the apparent Arrhenius equations where the activation parameters depended on temperature and conversion. The dependence of the logarithm of the reaction rate on temperature and conversion was described by the empirical polynomial to increase the accuracy of fitting and the AAE was calculated by the isoconversional method and the fitting of reaction rate and conversion. The AAE surfaces determined for the four examples of char combustion and gasification varied considerably and differently from each other with temperature and conversion. This provides new information for a better understanding of these processes and their kinetic models.
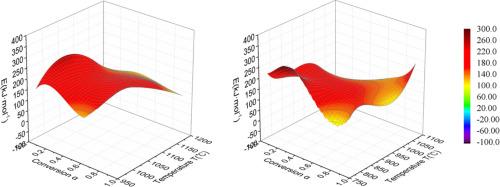
根据炭燃烧和气化的等温数据确定表观活化能面
本文利用木炭燃烧和气化的等温数据和表观阿伦尼乌斯方程确定了表观活化能(AAE)面,其中活化参数取决于温度和转化率。为了提高拟合的准确性,用经验多项式描述了反应速率对数与温度和转化率的关系,并通过等转化法和反应速率与转化率的拟合计算了 AAE。在四个炭燃烧和气化实例中测定的 AAE 面随温度和转化率的变化而有很大差异,且彼此不同。这为更好地理解这些过程及其动力学模型提供了新的信息。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Thermochimica Acta
化学-分析化学
CiteScore
6.50
自引率
8.60%
发文量
210
审稿时长
40 days
期刊介绍:
Thermochimica Acta publishes original research contributions covering all aspects of thermoanalytical and calorimetric methods and their application to experimental chemistry, physics, biology and engineering. The journal aims to span the whole range from fundamental research to practical application.
The journal focuses on the research that advances physical and analytical science of thermal phenomena. Therefore, the manuscripts are expected to provide important insights into the thermal phenomena studied or to propose significant improvements of analytical or computational techniques employed in thermal studies. Manuscripts that report the results of routine thermal measurements are not suitable for publication in Thermochimica Acta.
The journal particularly welcomes papers from newly emerging areas as well as from the traditional strength areas:
- New and improved instrumentation and methods
- Thermal properties and behavior of materials
- Kinetics of thermally stimulated processes
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


