Dependence of photocatalytic efficiency of titania-carbon nanotube nanocomposites on optoelectrical properties and colloidal stability
IF 4.1
3区 化学
Q2 CHEMISTRY, PHYSICAL
Journal of Photochemistry and Photobiology A-chemistry
Pub Date : 2024-10-20
DOI:10.1016/j.jphotochem.2024.116101
引用次数: 0
Abstract
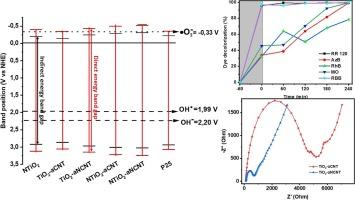
The correlation of photocatalytic efficiency of titania–amorphous (nitrogen doped) carbon nanotubes (TiO2–a(N)CNT) nanocomposites with colloidal and optoelectrical properties was investigated. TiO2–aNCNT and TiO2–aCNT exhibited narrow zeta potential variation, high polydispersity index, and large agglomerate sizes across the pH range 1–8. Despite their poor colloidal stability, TiO2–aNCNT exhibited superior degradation efficiency for high molecular weight reactive dyes, RR 120 and Remazol brilliant blue (RBB) with 55 mg.L−1 of RR 120 mineralized under solar irradiation. The photocatalytic performance for TiO2–aNCNT and TiO2–aCNT correlated with the respective energy band gaps 3.01 and 3.14 eV (respectively) and Ubarch tailing energies. Cyclic voltammetry showed a narrow peak-to-peak separation () of 200.7 V (vs Ag/AgCl) for TiO2–aNCNT thus confirming enhanced electron transfer kinetics. Electron impedance spectroscopy confirmed low charge transfer resistance for TiO2–aNCNT. Herein, it is demonstrated that optoelectrical properties are superior to colloidal stability in driving photocatalytic reactions.
二氧化钛-碳纳米管纳米复合材料的光催化效率与光电特性和胶体稳定性的关系
研究了二氧化钛-非晶态(掺氮)碳纳米管(TiO2-a(N)CNT)纳米复合材料的光催化效率与胶体和光电特性的相关性。TiO2-aNCNT和TiO2-aCNT在pH值为1-8的范围内表现出较小的zeta电位变化、较高的多分散指数和较大的团聚尺寸。尽管胶体稳定性较差,TiO2-aNCNT 对高分子量活性染料 RR 120 和雷马佐艳蓝(RBB)的降解效率却很高,在太阳光照射下,55 mg.L-1 的 RR 120 被矿化。TiO2-aNCNT 和 TiO2-aCNT 的光催化性能分别与各自的能带隙 3.01 和 3.14 eV 以及 Ubarch 尾能相关。循环伏安法显示,TiO2-aNCNT 的窄峰峰差(ΔEp)为 200.7 V(相对于 Ag/AgCl),从而证实了电子转移动力学的增强。电子阻抗光谱证实,TiO2-aNCNT 的电荷转移电阻较低。这表明,在驱动光催化反应方面,光电特性优于胶体稳定性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.90
自引率
7.00%
发文量
580
审稿时长
48 days
期刊介绍:
JPPA publishes the results of fundamental studies on all aspects of chemical phenomena induced by interactions between light and molecules/matter of all kinds.
All systems capable of being described at the molecular or integrated multimolecular level are appropriate for the journal. This includes all molecular chemical species as well as biomolecular, supramolecular, polymer and other macromolecular systems, as well as solid state photochemistry. In addition, the journal publishes studies of semiconductor and other photoactive organic and inorganic materials, photocatalysis (organic, inorganic, supramolecular and superconductor).
The scope includes condensed and gas phase photochemistry, as well as synchrotron radiation chemistry. A broad range of processes and techniques in photochemistry are covered such as light induced energy, electron and proton transfer; nonlinear photochemical behavior; mechanistic investigation of photochemical reactions and identification of the products of photochemical reactions; quantum yield determinations and measurements of rate constants for primary and secondary photochemical processes; steady-state and time-resolved emission, ultrafast spectroscopic methods, single molecule spectroscopy, time resolved X-ray diffraction, luminescence microscopy, and scattering spectroscopy applied to photochemistry. Papers in emerging and applied areas such as luminescent sensors, electroluminescence, solar energy conversion, atmospheric photochemistry, environmental remediation, and related photocatalytic chemistry are also welcome.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


