Codoping of carbon and boron composition in Na3V2(PO4)2F3 affects its sodium storage properties
IF 4.1
3区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
NASICON-type Na3V2(PO4)2F3 (NVPF) is a promising cathode material for Na-ion batteries due to its higher discharge capacity, appropriate voltage platform and output energy density. But its poor electronic conductivity should be increased to push its utilization. Here, the in-situ carbon and heteroatom B are introduced into to NVPF to improve the electrochemical performance. When citric acid is used as a carbon source, the free movement of electrons between V and the citric acid group is facilitated by the electrostatic force, causing a change in the valence state of V. It is a mixed valence of + 4 and + 3 for V in the product after annealing. The doping of B at O sites has no effect on ionic bond in solution and the crystal structure of the product. But, it decreases the formation energy, induces the charge redistribution and improves the conductivity. Excellent electrochemical performance is achieved with a B doping of 15 % to NVPF/C. Even after 1000 cycles, a capacity of 47.9 mAh g−1 is retained. The structure of NVPF/C-B is preserved during cycling, but compositional deviation at the electrode surface leads to some degradation. The relationship between the valence of V, doping B into the NVPF, and the degradation mechanism over repeated cycles provide a deep understanding of the complex interplay between metallic ions and carbon source, and the B-doping.
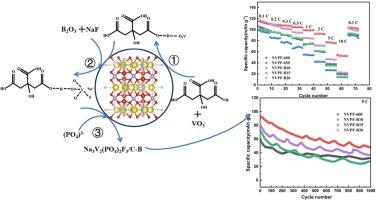
Na3V2(PO4)2F3 中碳和硼成分的共轭会影响其钠储存特性
NASICON型Na3V2(PO4)2F3(NVPF)具有较高的放电容量、适当的电压平台和输出能量密度,是一种很有前途的镎离子电池正极材料。但其电子传导性较差,应提高其利用率。在此,我们在 NVPF 中引入了原位碳和杂原子 B,以改善其电化学性能。当使用柠檬酸作为碳源时,静电力会促进 V 与柠檬酸基团之间电子的自由移动,从而导致 V 的价态发生变化。在 O 位点掺入 B 对溶液中的离子键和产品的晶体结构没有影响。但是,它降低了形成能,诱导了电荷的重新分布,并提高了导电性。在 NVPF/C 中掺入 15% 的硼后,电化学性能极佳。即使经过 1000 次循环后,仍能保持 47.9 mAh g-1 的容量。在循环过程中,NVPF/C-B 的结构得以保留,但电极表面的成分偏差导致了一些降解。通过研究 V 的价态、在 NVPF 中掺杂 B 以及反复循环过程中的降解机制之间的关系,可以深入了解金属离子、碳源和 B 掺杂之间复杂的相互作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.80
自引率
6.70%
发文量
912
审稿时长
2.4 months
期刊介绍:
The Journal of Electroanalytical Chemistry is the foremost international journal devoted to the interdisciplinary subject of electrochemistry in all its aspects, theoretical as well as applied.
Electrochemistry is a wide ranging area that is in a state of continuous evolution. Rather than compiling a long list of topics covered by the Journal, the editors would like to draw particular attention to the key issues of novelty, topicality and quality. Papers should present new and interesting electrochemical science in a way that is accessible to the reader. The presentation and discussion should be at a level that is consistent with the international status of the Journal. Reports describing the application of well-established techniques to problems that are essentially technical will not be accepted. Similarly, papers that report observations but fail to provide adequate interpretation will be rejected by the Editors. Papers dealing with technical electrochemistry should be submitted to other specialist journals unless the authors can show that their work provides substantially new insights into electrochemical processes.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


