Thermodynamic and molecular dynamics study of methane dry reforming
IF 5.6
2区 工程技术
Q2 ENERGY & FUELS
引用次数: 0
Abstract
The carbon neutrality strategy presents both challenges and opportunities for the metallurgical industry. Hydrogen, recognized as a green energy source, demonstrates significant potential for application in metallurgy. The negative impact of carbon deposition on catalysts is a significant challenge in the large-scale industrial application of methane dry reforming to produce hydrogen-rich reducing gases for ironmaking. This paper investigates the reaction mechanism through thermodynamic calculations and molecular dynamics simulations, systematically examining the effects of temperature, pressure, and feed ratio on the composition of gas products and the amount of carbon precipitation during the preparation process of hydrogen-rich reduction gas. The optimal conditions to produce high-quality reducing gas are identified to be a CO₂/CH₄ ratio of 0.8 at 1100K and 1 atm. At elevated temperatures, increasing the amount of carbon dioxide can reduce the amount of precipitated carbon, while the opposite is true at lower temperatures. The carbon absorbed by the nickel-based catalyst primarily originates from methane, while hydrogen ions activate carbon dioxide to produce carbon monoxide or carboxyl groups. By elucidating the reaction mechanism and quantifying the carbon precipitation, we provide theoretical guidance for industrial application.
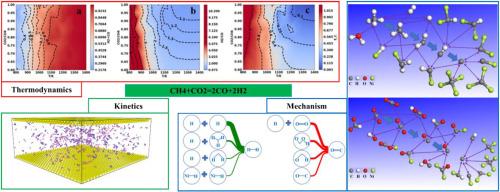
甲烷干转化的热力学和分子动力学研究
碳中和战略为冶金工业带来了挑战和机遇。氢是公认的绿色能源,在冶金领域的应用潜力巨大。碳沉积对催化剂的负面影响是大规模工业应用甲烷干重整生产炼铁用富氢还原气体的重大挑战。本文通过热力学计算和分子动力学模拟研究了反应机理,系统考察了富氢还原气制备过程中温度、压力和进料比对气体产物组成和碳析出量的影响。在 1100K 和 1 atm 条件下,CO₂/CH₄ 比率为 0.8,是生产高质量还原气体的最佳条件。在高温条件下,增加二氧化碳的量可以减少析出碳的量,而在低温条件下则相反。镍基催化剂吸收的碳主要来自甲烷,而氢离子激活二氧化碳产生一氧化碳或羧基。通过阐明反应机理和量化碳沉淀,我们为工业应用提供了理论指导。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of The Energy Institute
工程技术-能源与燃料
CiteScore
10.60
自引率
5.30%
发文量
166
审稿时长
16 days
期刊介绍:
The Journal of the Energy Institute provides peer reviewed coverage of original high quality research on energy, engineering and technology.The coverage is broad and the main areas of interest include:
Combustion engineering and associated technologies; process heating; power generation; engines and propulsion; emissions and environmental pollution control; clean coal technologies; carbon abatement technologies
Emissions and environmental pollution control; safety and hazards;
Clean coal technologies; carbon abatement technologies, including carbon capture and storage, CCS;
Petroleum engineering and fuel quality, including storage and transport
Alternative energy sources; biomass utilisation and biomass conversion technologies; energy from waste, incineration and recycling
Energy conversion, energy recovery and energy efficiency; space heating, fuel cells, heat pumps and cooling systems
Energy storage
The journal''s coverage reflects changes in energy technology that result from the transition to more efficient energy production and end use together with reduced carbon emission.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


