Dual surface-bulk aluminum modification in a-Al2O3@Na3VAl(PO4)3 sodium-ion batteries cathode to boost high voltage utilization and electrolyte protection
IF 8.1
2区 工程技术
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
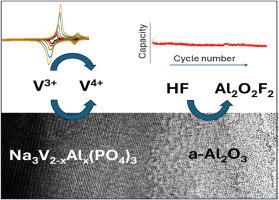
Amorphous alumina (a-Al2O3)-coated Na3VAl(PO4)3 samples are prepared by a low-cost, easily scalable, and environmentally friendly sol-gel process. X-ray diffraction reveals no significant structural changes after deposition. The presence of the amorphous carbon conductive and a-Al2O3 phases will be respectively confirmed in the light of Raman and NMR spectroscopies. Electron microscopy evidences the presence of alumina particles deposited on the substrate. Ex-situ XRD shows the reversible structural changes while sodium is inserted. Ex-situ XPS reveals the effective participation of V5+/V4+/V3+ species during the electrochemical reaction, while the formation of aluminum oxyfluorides justifies the efficient HF removal that prevents electrode degradation.
Electrochemical tests will validate this proposal. Thus, rate capability essays indicate that 1–3 % a-Al2O3 coating enhances capacity at high rates, with coated samples exhibiting a fast sodium migration and lower cell resistance at both the beginning and conclusion of cycling tests. It is supported by evaluating the kinetic response showing their high capacitive contributions and diffusion coefficients, especially in the 2 % a-Al2O3 coated sample. Eventually, these findings are corroborated by the good capacity retention of the 2 % coated sample during prolonged cycling at both room and low temperatures.
在 a-Al2O3@Na3VAl(PO4)3 钠离子电池正极中进行双表面包铝改性以提高高压利用率和电解质保护能力
通过一种低成本、易扩展且环保的溶胶-凝胶工艺制备了无定形氧化铝(a-Al2O3)包覆的 Na3VAl(PO4)3 样品。X 射线衍射显示沉积后的结构无明显变化。拉曼光谱和核磁共振光谱分别证实了非晶碳导电相和 a-Al2O3 相的存在。电子显微镜证明了基底上沉积的氧化铝颗粒的存在。原位 XRD 显示了钠插入时的可逆结构变化。原位 XPS 显示 V5+/V4+/V3+ 物种在电化学反应中的有效参与,而铝氧氟化物的形成则证明了高效的氢氟酸去除可以防止电极降解。因此,速率能力论文表明,1-3 % 的 a-Al2O3 涂层可在高速率下提高容量,涂层样品在循环测试开始和结束时都表现出钠迁移快和电池电阻低的特点。对动力学响应的评估也证明了这一点,动力学响应显示了高电容贡献和扩散系数,尤其是在 2% a-Al2O3 涂层样品中。最后,2% 镀膜样品在室温和低温下长时间循环测试时的良好电容量保持也证实了这些发现。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Power Sources
工程技术-电化学
CiteScore
16.40
自引率
6.50%
发文量
1249
审稿时长
36 days
期刊介绍:
The Journal of Power Sources is a publication catering to researchers and technologists interested in various aspects of the science, technology, and applications of electrochemical power sources. It covers original research and reviews on primary and secondary batteries, fuel cells, supercapacitors, and photo-electrochemical cells.
Topics considered include the research, development and applications of nanomaterials and novel componentry for these devices. Examples of applications of these electrochemical power sources include:
• Portable electronics
• Electric and Hybrid Electric Vehicles
• Uninterruptible Power Supply (UPS) systems
• Storage of renewable energy
• Satellites and deep space probes
• Boats and ships, drones and aircrafts
• Wearable energy storage systems
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


