Multicomponent synthesis of 7-(diethylamino)coumarin–pyrrolo[3,4-b]pyridin-5-one conjugates and modulation of their twisted intramolecular charge transfer (TICT) processes
IF 2.1
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
By coupling an Ugi-Zhu three-component reaction to a cascade sequence (aza Diels-Alder cycloaddition/N-acylation/decarboxylation/dehydration) into a one-pot process, seven new 7-(diethylamino)coumarin-pyrrolo[3,4-b]pyridin-5-one bis-heterocyclic conjugates were synthesized in moderate overall yields (15–38 %). Next, the photophysical properties of all products were determined using UV–Vis and fluorescence spectroscopy. These products exhibited fluorescence quantum yields (FQY) ranging from 17 % to 25 %. In contrast, one of their precursors, the free 7-(diethylamino)-3-carbaldehyde displayed a low FQY of 2 %, and, also exhibited dual emission across all solvents used for the measurements. The increase in quantum yield observed upon incorporation of the coumarin moiety into MCR products was attributed to the inhibition of the twisted intramolecular charge transfer (TICT) process. This one naturally occurs in the free coumarin but was suppressed in the products due to a decrease in the acceptor strength of the substituent at position 3 of the coumarin moiety. Furthermore, the electronic structure of the compounds was computed by DFT/TD-DFT calculations allowing to determine which molecular orbitals (MO) were involved in the electronic transitions.
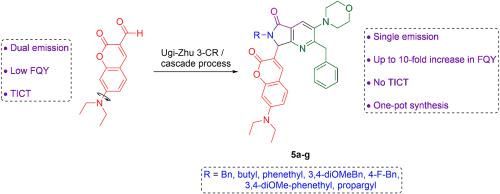
7-(二乙基氨基)香豆素-吡咯并[3,4-b]吡啶-5-酮共轭物的多组分合成及其扭曲分子内电荷转移(TICT)过程的调制
通过将 Ugi-Zhu 三组分反应与级联顺序(氮杂 Diels-Alder 环加成/N-酰化/脱羧/脱水)耦合为一锅工艺,合成了七种新的 7-(二乙基氨基)香豆素-吡咯并[3,4-b]吡啶-5-酮双环共轭物,总产率适中(15-38%)。接下来,使用紫外可见光谱和荧光光谱测定了所有产物的光物理特性。这些产物的荧光量子产率(FQY)在 17% 到 25% 之间。相比之下,它们的前体之一,游离的 7-(二乙基氨基)-3-甲醛的荧光量子产率较低,仅为 2%,而且在测量所用的所有溶剂中都表现出双重发射。在 MCR 产物中加入香豆素分子后,量子产率有所提高,这是因为分子内电荷转移(TICT)过程受到抑制。这一过程在游离香豆素中自然发生,但由于香豆素分子第 3 位取代基的受体强度降低,在产物中受到抑制。此外,还通过 DFT/TD-DFT 计算确定了化合物的电子结构,从而确定了哪些分子轨道(MO)参与了电子跃迁。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Tetrahedron
化学-有机化学
CiteScore
3.90
自引率
4.80%
发文量
439
审稿时长
34 days
期刊介绍:
Tetrahedron publishes full accounts of research having outstanding significance in the broad field of organic chemistry and its related disciplines, such as organic materials and bio-organic chemistry.
Regular papers in Tetrahedron are expected to represent detailed accounts of an original study having substantially greater scope and details than that found in a communication, as published in Tetrahedron Letters.
Tetrahedron also publishes thematic collections of papers as special issues and ''Reports'', commissioned in-depth reviews providing a comprehensive overview of a research area.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


