Synthesis and properties of β-arylated triphyrins[2.1.1] by successive introduction of one to six aryl groups at the β-pyrrole carbons
IF 2.1
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
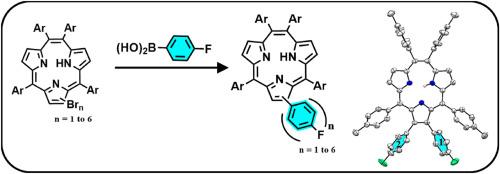
The β-arylated meso-tetra(p-tolyl) triphyrins[2.1.1] containing two, three, and four p-fluorophenyl groups were synthesized by reacting appropriate β-bromo triphyrin[2.1.1] with p-fluorophenyl boronic acid in toluene/THF/H2O (1:1:1) at 80 οC for 8 h–12 h and their properties were compared with our previously reported β-arylated triphyrins[2.1.1] containing one and six p-fluorophenyl groups. The increase of the number of p-fluorophenyl groups at the β-pyrrole carbons induces steric hindrance at the periphery of triphyrin[2.1.1] that results in the alteration of electronic properties due to distortion in the macrocycle which is reflected in their structure, spectral and redox properties. The X-ray structure analysis revealed that β-di(p-fluorophenyl) meso-tetra(p-tolyl) triphyrin[2.1.1] was almost planar like meso-tetra(phenyl) triphyrin[2.1.1] whereas the β-hexa(p-fluorophenyl) meso-tetra(p-tolyl) triphyrin[2.1.1] was significantly distorted. The absorption spectra of β-arylated triphyrins[2.1.1] showed bathochromically shifted Soret and Q-bands with reduction in absorption coefficients and increase in the bandwidth compared to the β-unsubstituted meso-tetra(p-tolyl) triphyrin[2.1.1] and the maximum effects were noted for β-hexa(p-fluorophenyl) meso-tetra(p-tolyl) triphyrin[2.1.1]. The β-arylated meso-tetra(p-tolyl) triphyrins[2.1.1] are stable under electrochemical conditions and DFT/TD-DFT studies are in agreement with the experimental observations.
通过在 β-吡咯碳原子上连续引入 1 至 6 个芳基,合成 β-芳基化三吡咯[2.1.1] 及其特性
通过将适当的β-溴三嗪[2.1.1]与对氟苯基硼酸在甲苯/THF/H2O(1:1:1)中反应8 h-12 h,合成了含有两个、三个和四个对氟苯基基团的β-芳基化中四(对甲苯基)三嗪[2.1.1]。1]与对氟苯基硼酸在甲苯/THF/H2O(1:1:1)中于 80 οC下反应 8 小时至 12 小时,并将它们的性质与我们之前报道的含有一个和六个对氟苯基基团的β芳基化三嗪[2.1.1]进行了比较。β-吡咯碳原子上对氟苯基基团数量的增加在三吡咯[2.1.1]的外围引起了立体阻碍,导致大环畸变而改变了电子特性,这反映在它们的结构、光谱和氧化还原特性上。X 射线结构分析表明,β-二(对氟苯基)中-四(对甲苯基)三聚吡啶[2.1.1]与中-四(苯基)三聚吡啶[2.1.1]几乎呈平面状,而 β-六(对氟苯基)中-四(对甲苯基)三聚吡啶[2.1.1]则明显扭曲。与 β-未取代的介-四(对甲苯基)三聚吡喃[2.1.1]相比,β-芳基化三聚吡喃[2.1.1]的吸收光谱显示出浴色偏移的索雷特带和 Q 带,吸收系数减小,带宽增大,β-六(对氟苯基)介-四(对甲苯基)三聚吡喃[2.1.1]的影响最大。在电化学条件下,β-芳基化介-四(对甲苯基)三嗪[2.1.1]是稳定的,DFT/TD-DFT 研究与实验观察结果一致。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Tetrahedron
化学-有机化学
CiteScore
3.90
自引率
4.80%
发文量
439
审稿时长
34 days
期刊介绍:
Tetrahedron publishes full accounts of research having outstanding significance in the broad field of organic chemistry and its related disciplines, such as organic materials and bio-organic chemistry.
Regular papers in Tetrahedron are expected to represent detailed accounts of an original study having substantially greater scope and details than that found in a communication, as published in Tetrahedron Letters.
Tetrahedron also publishes thematic collections of papers as special issues and ''Reports'', commissioned in-depth reviews providing a comprehensive overview of a research area.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


