Heterogeneous synthesis of benzo[f]indole-4,9-diones derivatives using sulfonic resin and photocatalytic study
IF 2.1
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
This research introduces a heterogeneous approach utilizing a resin-bound sulfonic acid catalyst (Dowex®-H) to synthesize benzo[f]indole-4,9-dione derivatives using β-enaminones systems and 2-bromonaphtoquinone. This method facilitates synthesizing a diverse range of compounds with varying substitution patterns, achieving yields of 88 %. The catalyst can be reused for up to five cycles without requiring reactivation. These compounds absorption spectra also show absorption (λmax) around 250–260 nm with a significant transition around 400 nm. Their photocatalytic potential was assessed by the model reaction of benzylamine photooxidation, achieving a yield of over 99 % within 36 h.
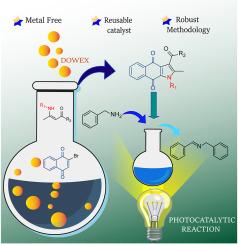
使用磺酸树脂异构合成苯并[f]吲哚-4,9-二酮衍生物及光催化研究
本研究介绍了一种利用树脂结合磺酸催化剂(Dowex®-H)的异构方法,使用β-烯氨基酮体系和 2-溴萘醌合成苯并[f]吲哚-4,9-二酮衍生物。这种方法有助于合成具有不同取代模式的多种化合物,产率达到 88%。催化剂可重复使用多达五个周期,无需重新激活。这些化合物的吸收光谱也显示出在 250-260 纳米附近的吸收(λmax),在 400 纳米附近有明显的转变。这些催化剂的光催化潜力通过苄胺光氧化模型反应进行了评估,在 36 小时内的产率超过 99%。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Tetrahedron
化学-有机化学
CiteScore
3.90
自引率
4.80%
发文量
439
审稿时长
34 days
期刊介绍:
Tetrahedron publishes full accounts of research having outstanding significance in the broad field of organic chemistry and its related disciplines, such as organic materials and bio-organic chemistry.
Regular papers in Tetrahedron are expected to represent detailed accounts of an original study having substantially greater scope and details than that found in a communication, as published in Tetrahedron Letters.
Tetrahedron also publishes thematic collections of papers as special issues and ''Reports'', commissioned in-depth reviews providing a comprehensive overview of a research area.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


