Facile electrooxidation of urea on nickel/metal oxide nanocomposites in alkaline media
IF 3.9
3区 材料科学
Q2 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
Development of efficient non-precious metal catalysts for electrocatalytic urea oxidation reaction (UOR) is highly sought to realize urea electrolysis as a viable electrochemical technology for the hydrogen production. Herein, a facile hydrothermal method is demonstrated to fabricate nickel/metal oxide nanocomposites (Ni-MnO2, Ni-TiO2 and Ni-MnO2/TiO2). The morphology and structural details of nickel/metal oxide nanocomposites are assessed using transmission electron microscopy (TEM) including high-resolution TEM, scanning electron microscopy (SEM)-energy dispersive spectroscopy (EDS) and X-ray diffraction (XRD). Electrochemical efficacies of nickel/metal oxide nanocomposites are evaluated in 0.35 M urea solution under alkaline conditions (1 M KOH) using cyclic voltammetry (CV) and linear sweep voltammetry (LSV). Among the studied catalysts, Ni-MnO2/TiO2 exhibits reasonable electrocatalytic activity towards UOR (1.29 V vs Ag/AgCl satd. KCl is required to achieve 10 mAcm−2 current density). Improved interfacing of nickel with tubular-like MnO2 and presence of TiO2 with MnO2 all together contributed for the observed higher UOR catalytic activity. This work demonstrates the efficacies of interfacial engineering in achieving high performing electrocatalysts for UOR.
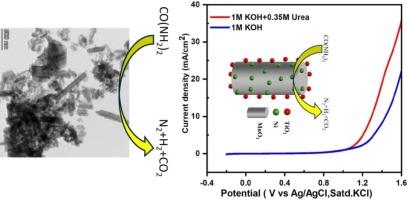
碱性介质中镍/金属氧化物纳米复合材料上尿素的简便电氧化反应
为了使尿素电解成为一种可行的制氢电化学技术,人们一直在寻求开发用于电催化尿素氧化反应(UOR)的高效非贵金属催化剂。本文展示了一种简便的水热法制备镍/金属氧化物纳米复合材料(Ni-MnO2、Ni-TiO2 和 Ni-MnO2/TiO2)。使用透射电子显微镜(TEM)(包括高分辨率 TEM)、扫描电子显微镜(SEM)-能量色散光谱(EDS)和 X 射线衍射(XRD)评估了镍/金属氧化物纳米复合材料的形态和结构细节。在碱性条件(1 M KOH)下的 0.35 M 尿素溶液中,使用循环伏安法(CV)和线性扫描伏安法(LSV)评估了镍/金属氧化物纳米复合材料的电化学效率。在所研究的催化剂中,Ni-MnO2/TiO2 对 UOR 具有合理的电催化活性(1.29 V 对 Ag/AgCl satd.达到 10 mAcm-2 电流密度需要氯化钾)。镍与管状二氧化锰界面的改善以及二氧化钛与二氧化锰的存在共同促成了所观察到的更高的 UOR 催化活性。这项工作证明了界面工程在实现高性能 UOR 电催化剂方面的功效。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Materials Science and Engineering: B
工程技术-材料科学:综合
CiteScore
5.60
自引率
2.80%
发文量
481
审稿时长
3.5 months
期刊介绍:
The journal provides an international medium for the publication of theoretical and experimental studies and reviews related to the electronic, electrochemical, ionic, magnetic, optical, and biosensing properties of solid state materials in bulk, thin film and particulate forms. Papers dealing with synthesis, processing, characterization, structure, physical properties and computational aspects of nano-crystalline, crystalline, amorphous and glassy forms of ceramics, semiconductors, layered insertion compounds, low-dimensional compounds and systems, fast-ion conductors, polymers and dielectrics are viewed as suitable for publication. Articles focused on nano-structured aspects of these advanced solid-state materials will also be considered suitable.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


