Mechanism of TiO2 stabilization and promotion of the synergistic zero-valent Fe‒Cu photocatalysis-persulfate degradation of phenol
IF 5.7
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Fe-Cu bimetal materials exhibit high catalytic degradation activity with more active sites, faster charge transfer efficiency and synergistic effects on redox pairs. However, it faces the problems of easy compounding and instability. Therefore, we designed and synthesized Fe-Cu/TiO2 composite catalysts and constructed a synergistic photocatalytic-persulfate degradation system. The phenol (50 ppm) degradation efficiency of 70 % Fe-Cu/TiO2 was 97.3 % after 30 min of reaction, which was 1.49 and 16.51 times greater than those of Fe-Cu and TiO2, respectively. This was attributed to the fact that mixing TiO2 and Fe-Cu not only effectively promoted Fe-Cu dispersion but also improved the number of active sites and catalytic degradation activity. Moreover, the photogenerated electrons generated by TiO2 could promote the valence transition between Fe-Cu, slowly releasing Fe2+ and Cu0 to realize the continuous activation of PDS and enhance the degradation activity. Quenching experiments and EPR results showed that the catalytic degradation process was dominated by the nonradical 1O2, with SO4·-, ·OH and ·O2- radicals interacting synergistically. Based on the characterization and experimental results, a synergistic degradation mechanism of Fe-Cu/TiO2 was proposed, which provides a new approach for pollutant degradation.
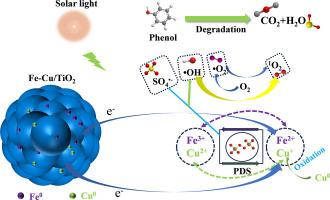
二氧化钛稳定和促进零价铁铜光催化-过硫酸盐协同降解苯酚的机理
铁铜双金属材料具有较高的催化降解活性,其活性位点多、电荷转移效率快,并能产生氧化还原对的协同效应。然而,它面临着易复合和不稳定的问题。因此,我们设计合成了 Fe-Cu/TiO2 复合催化剂,并构建了光催化-硫酸盐协同降解体系。反应 30 分钟后,70% Fe-Cu/TiO2 对苯酚(50 ppm)的降解效率为 97.3%,分别是 Fe-Cu 和 TiO2 的 1.49 倍和 16.51 倍。这是因为将 TiO2 和 Fe-Cu 混合在一起不仅能有效促进 Fe-Cu 的分散,还能提高活性位点的数量和催化降解活性。此外,TiO2 产生的光生电子可以促进 Fe-Cu 之间的价态转变,缓慢释放出 Fe2+ 和 Cu0,从而实现 PDS 的持续活化,提高降解活性。淬灭实验和 EPR 结果表明,催化降解过程以非自由基 1O2 为主,SO4-、-OH 和 -O2- 自由基协同作用。根据表征和实验结果,提出了Fe-Cu/TiO2的协同降解机理,为污染物降解提供了一种新方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Surfaces and Interfaces
Chemistry-General Chemistry
CiteScore
8.50
自引率
6.50%
发文量
753
审稿时长
35 days
期刊介绍:
The aim of the journal is to provide a respectful outlet for ''sound science'' papers in all research areas on surfaces and interfaces. We define sound science papers as papers that describe new and well-executed research, but that do not necessarily provide brand new insights or are merely a description of research results.
Surfaces and Interfaces publishes research papers in all fields of surface science which may not always find the right home on first submission to our Elsevier sister journals (Applied Surface, Surface and Coatings Technology, Thin Solid Films)
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


